Biochem 1 Flashcards
Major (non-enzymatic) protein functions
- Recognizing proteins:
ANY PROTEIN in a cell must have been ___ for by ___
Ultimately, all proteins are ___ products
- Must have been coded for by DNA
Ultimately, ALL PROTEINS ARE
GENE PRODUCTS
Carbohydrates
- Common disaccharides
- Lactose= ___+___?

LACTOSE=
galactose + glucose (ß-linked)
Vitamins & Minerals
- Define “MINERALS”
- What are 3 things theyre used for?
- How do you GAIN them?
- Are needed in Big/Small quantities?
MINERALS
Are inorganic elements or compounds
- Are necessary for:
- Bone formation
- ion gradients
- O2 transport, etc.
They are gained through: DIET
- Are needed in very small quantities
- which makes them “macronutrients”*
Protein Folding
- Hydrophobic surface:
The majority of the R groups on the surface of a globular protein are either ___ or ___ed
either POLAR or CHARGED
Substrate-Enzyme specificity
- The Enzyme-substrate (ES) complex is formed when?
- Show what the rxn looks like
is formed when substrate is bound to active site
E+S ⇔ES ⇔ EP ⇔ E+P

Protein Folding
- How do Salt Bridges form?
Formed when acidic & basic R groups undergo a NEUTRALIZATION rxn
- resulting in a salt
AA Rxns
- Protein hydrolysis
- TRYPSIN cleaves on the ____ side of WHAT AA’s?
Cleaves proteins on the CARBOXYL side of:
- Arginine and Lysine
What effect do ENZYMES (“Catalysts”) have on:
- Keq
- Yield
- % yield
NONE!!
LIPIDS are:
“Hydro_____ __________s”
“Hydrophobic Biomolecules”
Carbohydrates
- List the “8 Common Monosaccharides”
- glyceraldehyde
- dihydroxyacetone
- ribose
- deoxyribose
- glucose
- fructose
- galactose
- mannose
Enzyme Inhibition
- Feedback Inhibition
NEGATIVE FEEDBACK
- is what kind of inhibition?
- What does it do?
- What 3 things will you see it in?
NEGATIVE FEEDBACK
A specific type of non-competitive or
allosteric inhibition
- In it, one of the PRODUCTS of the reaction LATER in the chain
- …acts as an INHIBITOR for one of the enzymes EARLIER in the chain
Seen in:
- Multi-step reactions
-
Synthetic pathways
- e.g., GLYCOLYSIS
- Cascades

Major (non-enzymatic) protein functions
Immune system
- Name the 2 (GENERAL) types of proteins
AntiGENS & AntiBODIES
3º Protein structure
6 INTERACTIONS B/T AA’s that contribute to 3º structure
-
H-bonding
- Are ___-_____ bonds between WHAT 2 THINGS?
NON-COVALENT bond between either:
-
Backbone atoms
- N-H or
- C=O
-
Side chains
- Amine groups
- Carboxyl groups
- Alcohol groups, etc.
Enzyme Inhibition/Reversible Inhibition/Competitive inhibition does what? Effect on Vmax and Km
inhibitor binds AT the active site, and inhibitor resembles substrate in shape. Can be overcome by [S]. Vmax=NO ∆. Km=INCREASES.
PEPTIDES are

WRITTEN, READ, & SYNTHESIZED
from the ___ to ___ terminus
N to C
Lipids/Triaglycerols/ Saturated vs Unsaturated. Compare. Which is healthier? Why?
Saturated=no DBs, solid @ RT, Higher MPs. Unsaturated=at least 1 DB, liquid @ RT, Lower MPs). Unsaturated is healthier b/c they generate fewer calories when metabolized.
Mechanisms of Catalysis
- What are COFACTORS?
- What 2 things qualify as Cofactors?
General term for any species that is:
- required by an enzyme to function
Coenzymes and Prosthetic groups are both cofactors
Draw a mechanism for:
SULFUR LINKAGE OF TWO CYSTEINES

Enzyme classification by rxn type
- What kind of reactions do TRANSFERASES participate in?
- Describe and give an example
transfer of an R group
Example: Kinases, aminotransferases
Carbohydrates/Carbohydrate Rxns/ Hydrolysis of Glycoside linkage
Polymer (n) + H2O–>Polymer (n-1)+monomer
Protein Separation Techniques/Electrophoresis: describe the experiment.
Used to separate by size. Proteins denatured by SDS, are given a uniform (-) charge. Gives protein uniform q/m ratio. Bigger proteins are found at the top of the gel, and smaller proteins move further towards the bottom.
Michaelis-Menten Kinetics/Lineweaver-Burke Plots/y-intercept=?
y-intercept= 1/Vmax
Protein structure/2º/alpha sheets: H-bonding b/t ___ and ___ that are exactly ___ residues apart. What else is involved in H bonding? Where are R groups directed?
b/t carbonyl O’s and amide H’s that are exactly 4 residues apart. ONLY every 4th residue is involved in H bonding.R groups directed towards outside of cynlinder.
Mechanisms of Catalysis/Simple proteins. If an enzyme is a simple protein, what can it also be called?
are proteins that contains only AAs and NO non-protein cofactors or prosthetic groups. If a simple protein is an enzyme, it’s called an “apoenzyme”
What important thing should you remember about ZWITTERIONS?
HINT: WHAT IS THE CONNECTION B/T ZWITTERIONS, AA’S AND pH?

ALL of the amino acids exist as
- Zwitterions at a pH of 7.4*
- With the EXCEPTION of amino acids that have charged –R groups (Asp, Glu, Lys, Arg, His)
This can be very confusing because textbooks NEVER draw them this way!
- Most texts draw them in their “non-ionized” form
- with –COOH and –NH2 groups
That combination DOES NOT EXIST!
at physiological pH
…or at ANY pH!
Below a pH of about 9 the amine group will get protonated:
- -NH3+
Above a pH of 9 the amine group will be –NH2
- (as is shown in most texts)
- …But at that very high pH (>9) the carboxyl group will have LONG AGO been deprotonated!*
- would be -COO- at a pH ~ 2

Carbohydrates/Carbohydrate Rxns/ Keto-enol tautomerization is an equilibrium b/t what two things? What are tautomers of e/o?
equilib b/t a keto form (a ketone or an aldehyde) and an enol (alcohol). Enol and Keto are tautomers of e/o.
What is a ZWITTERION?
- Give an example
a DIPOLAR VERSION of an AA
- wherein positively and negatively charged R groups CANCEL EACH OTHER OUT!
- Results in a NEUTRAL ion
Example:
- Isoleucine
- Draw a Fischer projection of the amino acid alanine in both its L- and D- forms*
- Which of the two forms is predominant in nature?*
- D – and L- amino acids are MIRROR IMAGES of one another*
- but they are NOT IDENTICAL compounds*
Think of your left and right hands
- They are mirror images
- but you cannot superimpose one upon the other
- because they are arranged in a fundamentally different way
- but you cannot superimpose one upon the other
L – amino acids
are predominant in nature
Although a few D – amino acids are used by some bacteria

Draw a mechanism for:
HYDROLYSIS OF A PEPTIDE BOND BETWEEN GLYCINE AND ALANINE

How can you tell if a substrate will bind in an active site?
- Depends on:
- the complementary charges on R groups and/or
- hydrophil/phobicity of the R groups
Carbohydrates/what are the 2 types? What MFs to they have?
1) Monosaccharides (CH2)n.2) Disaccharides Cn(H2O)x.
Carbohydrates/Carbohydrate Rxns/ Polymerization: ___+___=?
monosaccharides + disaccharides=polysaccharides
Protein structure/3º: List the 6 molecular interactions that contribute to 3º structure?
1) H-bonding. 2) DSB’s. 3) Hydrophobic/philic interxns. 4) Ionic interxns. 5) VDWs. 6) Proline turns.
Protein structure/2º/Beta sheets: H-bonding b/t what? Where are the R groups located? What shape do beta sheets have? What does this serve?
H-bonding b/t ALL carbonyl O’s and the amide H’s in the adjacent row. R groups are perpendicular to the plane of the beta sheet, on both sides.Beta sheets have PLEATED conformation. THis lines carboxyl & amide regions up so that each residue is participating in 2 H bonds.
Two theories of enzyme specificity/Lock & Key model
enzyme to substrate is an EXACT FIT (not favored by scientists)
Enzyme Inhibition/Irreversible Inhibition: how does the inhibitor bind? What effect does this have?
Inhibitor binds COVALENTLY to enzyme and/or the active site, disabling the enzyme for either a long time or permanently
Michaelis-Menten Kinetics/MM constant (Km)= relative measure of what? What is Km equal to?
measure of an enzyme affinity for its substrate. Km=[S] at 1/2 Vmax
Michaelis-Menten Kinetics/MM equation=? Shows relationship b/t?
v=Vmax[S]/(Km+[S]). Shows relationship b/t rxn velocity, Km, and [S].
What does it mean when pH is lower than pI?
it means the molecule has a (+) pI value
Mechanisms of Catalysis/Conjugated proteins. Define & give an example. If an enzyme is a conjugated protein, what is it called?
=a protein that is associated with its cofactors, either covalently or via IMFs. Ex: Hb (which has its NP Heme group). If its a conjugated protein thats an enzyme, its called a “holoenzyme”
Carbohydrates
Glucose Polysaccharides
- What is STARCH?
- What is it found in, and what is it used for?

branched, α-linked (“alpha-site” side)
- glucose polymer*
- used for energy storage in PLANTS
Protein Folding
- Entropy & Protein Folding:
Transition from solvation of ___-_____regions to solvation of ___ or ___ed globular protein surface results _______ed ENTROPY
- Transition from solvation of NONPOLAR regions to*
- solvation of POLAR or CHARGED globular protein*
- surfaces results in INCREASED entropy*
- Even when water interacts with a dissolved polar solute, this interaction is less entropically favorable that those same water molecules interacting with only other water molecules
- However, the driving thermodynamic force that favors protein folding results from the fact that non-polar regions require a much GREATER ordering of water molecules to accomplish solvation
- Therefore, transitioning from solvation of non-polar regions to solvation of a mostly polar or charged globular protein surface represents a net increase in entropy*
- In fact, it is enough to overcome the decreased entropy associated with the protein being in a folded rather than an unfolded state
This favorable increase in entropy is a major contributor to the overall conformational stability of the folded protein
- Each AA has a minimum of __ acidic protons, which are?
- Do ALL AAs have this many?
2 acidic protons
-COOH and -NH3+
- 7 AA’s have acidic R groups
- So they have 3 acidic protons in total
Feedback Inhibition
-
Phosphorylation:
- ….Is the addition of what?
- What puts it there?
Ph group added to a molecule
- by a KINASE (Which is a type of TRANSFERASE)
Draw a mechanism for:
STRECKER SYNTHESIS OF ALANINE

Protein Folding
-
Electrostatic Interxns:
- Are interactions between WHAT?
- What 2 things do these interactions do?
are interactions between CHARGED R GROUPS
Functions:
- Encourage the ACT of folding
- STABILIZE the protein once it IS folded
Carbohydrate Rxns
- What happens during “RING CLOSING?”
INTRAmolecular Nucleophilic substitution

(aka is all happening within the same ring-containing molecule)
Here, the -OH group
- (of the chiral C that is FURTHEST from the carbonyl C)
- acts as a NUCLEOPHILE–
- Attacking the (ELECTROPHILIC) carbonyl C
- Carbonyl Oxygen is then protonated to form a -OH group
- Attacking the (ELECTROPHILIC) carbonyl C
Protein Folding/ Protein denaturing: name the 4 protein denaturing agents.
1) Heat. 2) Acid. 3) Urea. 4) Mercaptoethanol.
Carbohydrates
Glucose Polysaccharides
- Describe GLYCOGEN
- What organisms use it, and what for?
- How does it compare to STARCH?

branched, α-linked (α 1,4/1,6)
glucose polymer
used for energy storage in ANIMALS
vs. Starch:
- Both used for energy storage
- Starch is found in PLANTS, though
- Both have same (α 1,4/1,6) linkages
- Starch is 80% amylopectin (branched) and 20% amylose (UNbranched)
Glycogen is 100% amylopectin, thus is MORE BRANCHED THAN STARCH!

Carbohydrates/Cyclic Structure & Conformation of hexoses/Hemiacetals vs Hemiketals
Hemiketal (R,R,OH,OR). Hemiacetal (R,H,OR,OH)
Major (non-enzymatic) protein functions/Structural Proteins: Name the 4 kinds, and what they are found.
1) Actin [thin filaments, microfilaments]. 2) Tubulin [MT’s]. 3) Keratin [IMFs]. 4) Elastin [collective tissue, ECM].
- All native AAs are L or D?
- Are L,D and R,S the same?
- all native AAs are L
- L,D NOT directly correlated with R,S
- Should be considered separate
- Most L AAs are S
- but some are R
- e.g. cysteine
- but some are R
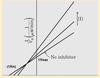
Enzyme Inhibition/Reversible Inhibition/Non-competitive inhibition does what? Effect on Vmax and Km
Inhibitor binds AWAY from active site and ∆es shape of the enzyme. Inhibitor has equal affinity for both the ES complex and the enzyme. Vmax=DECREASES. Km=NO ∆.
Michaelis-Menten Kinetics/Lineweaver-Burke Plots/x-intercept=?
x-intercept= - 1/Km
WRT STEREOCHEMISTRY, what do all AAs (except for ____) have in common?
- What 4 different substituents does each AA have?
An alpha-C stereocenter:
- all AAs (except for GLYCINE ) are CHIRAL at the α-carbon
4 *DIFFERENT* substituents:
- R group
- an H
- a COOH
- an NH2
Protein Folding/ Solvation Layer: what is it and what interacts with what?
is a layer of H2O that surrounds a dissolved protein. H2O’s in the layer interact with w/o and with protein’s surface.
Protein structure/3º/6 binding forces/DSB’s
covalent bond b/t the Sulfurs (or Seleniums) of 2 Cysteine residues.
Carbohydrates/Stereochemistry/L-sugars
L sugars do NOT occur naturally in humans
Carbohydrates/Carbohydrate Rxns/ Polymerization/Glucose Polysaccharides/Cellulose
ß-linked glucose polymer, used for energy storage in plants (like starch), is INDIGESTIBLE to animals w/o some form of symbiotic bacteria
Describe FISCHER PROJECTIONS:
- What do the horizontal & vertical lines represent?
- Fischer Projections CAN be rotated ___°, but CAN’T be rotated ___° or ___°
EXPLAIN WHY YOU CAN’T ROTATE THE MOLECULE IN CERTAIN WAYS

A Fischer projection is a representation of a 3D molecule drawn in 2Ds
- A tetrahedral carbon is represented as two crossed lines
- and the groups attached to that carbon are displayed
-
The HORIZONTAL line
- is extending “OUT” of the paper
- Toward you
-
The VERTICAL line
- is BEHIND the plane of the paper
- Away from you
Because of this, Fischer projections
CAN be rotated 180°
but not 90° or 270°
180° rotation just flips the molecule over:
- the same R groups are extending forward or backward
But if you rotate the molecule just 90° in
either direction:
- you have CHANGED which R groups are above or below the plane of the paper
...which changes the stereochemistry of the molecule
Absolute configuration
- All AAs are what?
- What determines this?
Either L or D
- depending on which side the NH2 group is located in a Fischer Projection
- L=on the left
- D=on the right
Isoelectric point is similar to the ___ ___ in acid-base titration. Why?
to the equivalence point. Both are in the middle of their respective titration curves.
Carbohydrates/Stereochemistry/How are D-galactose and L-galactose related? What do the D and L represent?
They’re ENANTIOMERS (same molecule, different stereochemistry at last chiral C). In Fischer projections, the furthest -OH group from the carbonyl is to the LEFT for L, to the RIGHT for D
Enzyme Inhibition/Feedback Inhibition/ Zymogens are what? Why are they useful? What is an example?
are an inactive enzyme precursor. Useful b/c they can get activated quickly if needed, but are deadly if left on 24/7. Ex: Prothrombin (in blood coagulation).
What is an essential AA?
an AA that your body cannot synthesize. Must be ingested.