5. Atomic Methods - Emission Flashcards
1
Q
how does emission in atomic absorbtion work?
A
uses the light from a heated sample as a source of information
2
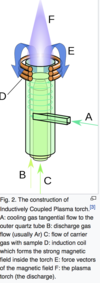
Q
what is the most common source of heat for atomic emission?
A
inductively coupled plasma
3
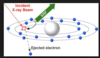
Q
what is x-ray fluorescene? give 4 points about it
A
a type of atomic emission
Core electrons can be excited by X-rays
Electrons relax by emission of X-rays
Energy analysis reveals the type of element emitting
Energy of X-rays can be measured using a solid state detector
(no monochromator)
4
Q
A


