4: testing materials Flashcards
(48 cards)
properties of ceramics
hard - difficult to scratch brittle - easily shatter into pieces due to rigid structure stiff - difficult to stretch or bend due to strong bonds between atoms.
describe structure of a ceramic
the ceramics are either ionically or covalently bonded in a giant rigid
strong bonds between atoms make ceramics stiff.
however there is no pattern in its structure, the atoms are arranged randomly. this random atomic bonding means there are no slip planes in its ceramic lattice, as well as no mobile dislocations this makes ceramics very brittle.
being brittle is why cracks spread through them when they fracture, because the applied force acts over a very small area so the stress is high
PIC properties with desc of metals
some are malleable (can be shaped easily) and some are ductile (can be drawn into wires) this is due to the dislocations (missing atoms in metal structure) allowing planes (rows) of metal atoms to slip over each other when a force is applied
some are stiff - strong metallic bonds between ionic lattice and delocalised electrons
good conductors - metals have a sea of delocalised electrons that allows metals to conduct electricity
tough: it absorbs a lot of energy (deforms plastically) before fracture. this is because when you apply a stress the metal deforms plastically in the region of the crack, making the crack broader reducing stress around it
PIC structure of metals
crystalline metallic lattice - atoms are arranged in a regular repeating pattern
PIC what happens when you apply a force to a metal with dislocation
planes of metal atoms slip over each other
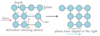
PIC what happens when you put the atoms of a second metal in dislocations what is this process called what’s the effect
atoms of a second metal (impurities) can be placed inside dislocations to pin them down, increasing the stress needed to cause slipping this is alloying this causes metals to be harder and less ductile
what is a perfect metal
a metal with no dislocations (missing atoms)
what is a polymer 2 types of polymers
a polymer is a molecular chain, made up of single repeating units called monomers there are man made polymers and natural polymers
example of polymer
rubber, sulfur atoms form cross link with polymer chains, the more sulfur = more cross links so more stiff polymer
PIC structure and bonding of a polymer
the monomers in a chain are covalently bonded so they’re very hard to separate polymer chains are often entangled but can be unravelled by rotating about their bonds when you pull them. this makes polymers flexible. the strength and number of bonds also affects the polymer’s flexibility. if the cross link bonds (chains tied at regular interval) are stronger and you have more of them, the more rigid the polymer

what is hooke’s law equation what is the constant a measure of,
for small extensions, the force F is proportional to the extension x F = kx F = force in newtons x = extensions in metres K = constant of proportionality (spring constant) Nm-1 the spring constant is a measure of stiffness, small when small force gives big extension, big when big force gives small extension
PIC what does the gradient of an extension force graph give you WARNING
the gradient for an extension force graph is the spring constant k k = f/x to get the gradient, sometimes it can get switched around by q’s so do 1 / gradient
PIC PRACTICAL: force extension graph for a rubber band explain the process
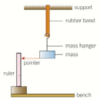
start with the shown apparatus, use a stiff wire wrapped around the bottom of the mass to provide a pointer measure the unstretched length of the band and then measure the length of the band with each mass you add on find the extension by subtracting old length from new length proceed to plot an extension against force graph
what is material compression what is the force called when you stretch a material
when you squash a material instead of stretch it if forces stretch the material they’re tensile
what is elastic deformation for a wire
when you deform a wire but it returns to its original length when force (stress) is removed
PIC what is the elastic limit E
the point up until an object elastically deforms
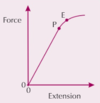
what is plastic deformation for a wire
a wire that has been permanently deformed and will not return to its original length when the stress (force) is removed
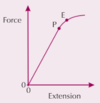
PIC what is the fracture stress B
the point after plastic deformation at which the wire breaks

PIC what is the limit of proportionality P
the point before the elastic limit where the graph is straight and extension is proportional to the force
what happens when you stretch a material
you store energy in it
2 equations to calculate energy stored in a spring
E = 1/2 Fx E = 1/2 Kx(x) = E = 1/2Kx²
what does the area under an extension force graph give when elastically deforming
when a body deforms elastically the energy stored is equal to the energy transferred stretching the spring the energy transferred stretching a material can be found from the area under the line
what is stress equation units
the force applied (tension) divided by cross sectional area (force per unit area) stress σ = tension / cross sectional area Nm-2
PIC what is the yield stress
the stress at which a material begins to deform plastically and become permanently deformed












