3.1.3 Lipids - Triglycerides, Phospholipids and the Emulsion Test Flashcards
Which 3 elements do lipids contain?
Carbon
Hydrogen
Oxygen
Which part of a phospholipid is hydrophilic?
The phosphate ‘head’
Identify the molecule
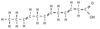
Polyunsaturated fatty acid

What does hydrophobic mean?
water hating
How does the structure of triglycerides relate to their properties?
- High ratio of carbon-hydrogen bonds make them an excellent energy source
- Low mass to energy ratio- animals don’t have to carry a heavy energy store around.
- Non-polar molecules are insoluble in water so they do not affect osmosis in cells.
- High ratio of hydrogen to oxygen atoms so water released when trigylcerides are oxidised. Good for animals in deserts.
Identify the molecule

Monounsaturated fatty acid

Identify the molecule
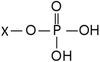
Phosphate molecule

What state do monounsaturated and polyunsaturated fats take at room temperature?
Liquid - they are oils.
What is mean by a saturated fatty acid?
A fatty acid that contains no double bonds between the carbon atoms
Identify the molecule

Glycerol

Phospholipids are p___________ molecules
polar
What does the following describe:
- Add 2cm3 of food and add to a test tube.
- Add 5 cm3 of ethanol
- Shake the tube thoroughly to dissolve any lipid.
- Add 5cm3 of water and shake gently
- A cloudy-white colour indicates a positive result.
The emulsion test for lipids
What state do saturated lipids take at room temperature?
Solid - they are fats
What is meant by a polyunsaturated fatty acid?
A fatty acid that contains more than 1 double bond between its carbon atoms.
What does hydrophilic mean?
attracted to water
What type of bond forms between glycerol and fatty acid molecules?
Ester bond
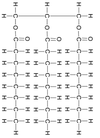
Identify the molecule

Triglyceride
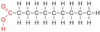
Identify the molecule

Saturated fatty acid
What type of reaction takes place when a fatty acid molecule is removed from a glycerol molecule?
Hydrolysis
What type of reaction takes place when a glycerol molecule joins with a fatty acid molecule?
Condensation reaction
Describe how you would carry out an emulsion test for lipids
The emulsion test for lipids:
- Add 2cm3 of food and add to a test tube.
- Add 5 cm3 of ethanol
- Shake the tube thoroughly to dissolve any lipid.
- Add 5cm3 of water and shake gently
- A cloudy-white colour indicates a positive result.
Which part of a phospholipid is hydrophobic?
The fatty acid ‘tails’
What decides the properties of different fats and oils?
Variations in their fatty acid chains
List the roles of lipids in living organisms.
- Cell membranes
- Energy store
- Waterproofing
- Heat insulation
- Electrical insulation
- Protection of organs e.g. kidneys
Why are triglycerides called triglycerides?
They contain 1 glycerol molecule (glyceride) and 3 fatty acids (tri)
What is meant by a monounsaturated fatty acid?
A fatty acid that contains 1 double bond between 1 pair of its carbon atoms.
Describe the structure of a phospholipid
1 glycerol molecule attached to
2 fatty acid molecules and
1 phosphate molecule
How does the structure of phospholipids relate to their properties?
- Polar molecules - meaning that they can form a barrier (cell membrane) between water inside and outside a cell.
- The phospholipid can form glycolipids by combining with carbohydrates. These are important in cell recognition.
Name the 2 main groups of lipid
Triglycerides (fats and oils)
Phospholipids
What happens when a phospholipid is placed in water?
The hydrophilic head positions itself towards water.
The hydrophobic tail positions itself away from water
Identify the molecule

Phospholipid
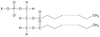
List the characteristics shared by all lipids
- They contain: Carbon, Hydrogen and Oxygen
- Lower proportion of oxygen to carbon and hydrogen than carbohydrates.
- Insoluble in water
- Soluble in organic solvents e.g. alcohols


