15 - Oxygen Transport Flashcards
What is the structure of haemoglobin?
- Quaternary structure
- 4 subunits, 2a 2b, tetrameric
- Each polypeptide chain has haem prosthetic group
- Each chain similar to myoglobin

What is the structure of the haem group?
- Porphyrin planar ring with Fe in the middle
- Free haem, Fe can make two bonds, one above and one below ring
- Forms bond with histidine residue (proximal histidine) from protein, and one molecule of O2
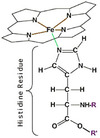
What does it mean by prosthetic route in haem?
When Fe is bound to a histidine residue and one O2 rather than two O2’s
What is the structure of myoglobin?
- Tertiary structure
- 1 subunit
- Mainly alpha helices (no beta)
- Compact (globular)
- Small
- His 93 in 8th alpha helix is covalently linked to Fe

What happens to the shape of myoglobin when oxygen binds?
- Fe is below the planar ring in deoxymyoglobin
- When oxygen binds this causes movement of Fe into the plane of the ring, causing Histidine to move up so very small change in protein conformation when histidine moves
- Not enough change to notice

What does the binding curve of myoglobin look like?

What does the haemoglobin binding curve look like?

What are the two states of haemoglobin?
T (tense) state: low affinity state, no o2 bound
R (relaxed) state: high affinity state, o2 bound
When O2 is bound it promotes stabilisation of the R state

What is the cooperative binding of oxygen?
As oxygen binds to haemoglobin, there is a conformational change in haemoglobin and this causes haemoglobin to have a higher affinity for O2
BINDING AFFINITY INCREASES AS MORE O2 BINDS

How does binding of oxygen to haemoglobin cause a change in conformation
Histidine pulling
Why is the cooperative effect important?
So oxygen binds at high partial pressure of oxygen and dissociates at low partial pressures of oxygen

What are the three ways oxygen binding is regulated?
1. 2,3-BPG (2,3 bisphosphate glycerate)
2. CO2 and H+
3. Carbon monoxide
What does 2-3 BPG do?
- Allosterically binds to haemoglobin, stabilising the T form
- Binds to positives on B-subunits
- Decreases affinity for O2 and shifts curve to the right
- In RBC at 5mM

How does 2,3-BPG concentration vary?
- In highly metabolic tissues high BPG
- At high altitudes more BPG produced
- More BPG promotes oxygen release into tissues
What is the Bohr effect?
- An increase in PCO2 OR a decrease in pH, causes a Hb to have a decrease in affinity for O2, curve shift to right
- Allosteric binding
- High metabolic tissues produce lots of H+ and CO2 so ensures oxygen release into tissue so supply can meet demand

How is carbon monoxide a poison?
- Binds very tightly to Hb and irreversible
- When bound to Hb it increases the affinity for O2 so other subunits can’t release their oxygen into tissue
- Fatal when over 50% COHb

What are the different types of haemoglobin and why are they different?
Different haemoglobins due to the different expressions of different polypeptide chains throughout life
Where are the alpha and beta subunit genes located?
A (two genes) - chromosome 16
B (only 1 gene)- chromosome 11
What haemoglobin is in fetuses?
HbF
a2γ2
Higher binding affinity for O2 than HbA so allows transfer of O2 from mother across the placenta

How do you treat carbon monoxide poisoning?
Hyperbaric chamber or 100% O2 in rebreather mask

What is sickle cell anaemia caused by?
- Subsitution mutation in beta Hb (Glu –> Val)
- Val hydrophobic not hydrophilic like Glu
- Hb clump together to hide hydrophobic amino acid and become insoluble, stick to RBC membrane (sickling)
- Prone to lysis as more rigid

Outline the relationship between haemogloin curve, affinity and shape of haemoglobin?
- Shift to right, decrease in affinity, T state
- Shift to left, increase in affinity, R state
What is Thalassaemia?
Genetic disease where there is an imbalance in alpha and beta chains so decrease in haemoglobin production
What is b-thalassemia?
- Decrease or absent b-globin chain
- Onset at birth-age 2
- a-chains can’t form stable tetramer

What is a-thalassemia?
- Decreased or absent a-globin chain
- Different levels of severity due to two alpha genes
- B chains can form stable tetramers but they have really high affinity for oxygen as no cooperativity
- Intolerance to exercixse
- Onset before birth

What is HbH disorder?
- It is a moderate-severe anaemia caused by alpha-thalassemia.
- Autosomal recessive disorder
Which haemoglobin has the highest affinity for 2,3BPG?
HbA, different side chains that BPG binds to


