Week 1: Pharmacodynamics I Flashcards
(30 cards)
What is pharmacodynamics?
What the drug does to the body
Drugs interact with specific receptors to cause a response:
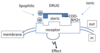
Drug (epinephrine) + Receptor (adren. receptor) <=> Drug/Receptor => Response
What type of biochemical interaction is similar to pharmacodynamics? What is the principal equation for pharmacodynamics?
The Michelis-Menten principal of enzyme kinetics is similar to the drug-receptor principle of pharmacodynamics.

What is the constant that dictates the drug/receptor interaction in pharmacodynamics, and what constant is it similar to?
Kd, or the dissociation constant, and it is similar to Km for enzymes
As you add more of a drug to a pharmacodynamic equation, what happens?
The response increases via
D + R(eceptor) <=> DR => Response
As the equation shifts to the right
What was Paul Erlich responsible for in pharmacodynamic chemistry?
The lock-and-key concept of drug/receptor interactions
What was John Langley’s key discovery regarding pharmacodynamics?
He found that nicotine activated skeletal muscle tissue, generating the concept of a receptive substance.
By what mechanisms do drugs combine with protein binding sites?
Drugs combine via precise physiochemical and steric interactions between chemical groups on the drug and chemical groups on the protein.
Where does binding energy for the drug/receptor complex come from?
Bonds and interactions (pHHILES or “files) including:
Lipophilic
Hydrophilic
Ionic
Steric
Hydrogen bonds
Electronic effects
pK effects (acidity values)
Can you have multiple types of binding in a drug/receptor interaction? If so, explain.
Yes–drugs are long molecules, often with several key interaction sites. Pictured is a drug that uses both lipophilic and ionic bonding properties to fit into a given receptor.
Why do receptors exist? What are some examples of things that interact with them?
Receptors that drugs interact with don’t exist BECAUSE of the drugs–rather, they exist due to the need for interactions of different body elements with endogenous ligands.
Opiate receptors are activated by endorphins and enkephalins produced by the body, and can also be stimulated by morphine, an exogenously produced substance (made from poppies!)
What are the five assumptions of drug-receptor interactions?
1) The binding of a drug to the receptor causes an event that leads to a response
2) The response to the drug is graded–i.e. dose-dependent (creates an exponential curve where Emax is analagous to Vmax)
3) The drug-receptor interaction follows mass-action relationships
4) For a given drug, the response is directly proportional to the bound vs unbound ((DR)/(RT)) receptor sites
5) The number of drug molecules is much greater than the number of receptors available
What are the two ways in which conformational changes occur in receptors?
Drugs either:
1) Cause a conformational change in the receptor
or
2) Bind to a preferred conformation of the receptor, as receptor conformations are continually in flux
How do mass-action relationships work?
More of a drug causes the reaction to shift to the right in:
D + R <=> DR => Response
This is reversible when there is one D/R interaction, but is not always reversible when there are multiple drug interactions that lead to a response.
What is the equilibrium dissociation constant for a drug/receptor interaction?
Kd = [D] * [R]
[DR]
How does the proportionality of bound and unbound receptors compare to the response curve? What is the term for a drug “taking up space” by interacting with a receptor?
(DR)/(RT) ∝ E/Emax
(∝ means “is proportional to”)
A drug interacting with a receptor, when bound, is called an “occupant”
How does the number of available drug molecules binding to receptors impact the amount of “free drug” available?
Because there are SO many more drug molecules than receptors, [R] goes down as drug molecules begin to bind, forming [DR]. However, [D] does NOT decrease significantly!
What is fractional occupancy? What does it depend on?
Fractional occupancy is the ratio of occupied vs free receptors:
[DR]/[RT] = [D]/KD+[D]
The fraction of receptors occupied depends ONLY on the concentration of the drug and it’s KD value
What are the effects of a given drug dependent on, and what is the relationship between this effect and a dose of a given drug?
The biological response, or effect, e is proportional to the fractional occupancy ([DR]/[RT]) and the maxiumum response, Emax is proportional to RT via:
e/Emax = [D]/KD+[D]
What kind of curve is created by plotting the response versus drug concentration from the e/Emax equation? How is it graphed or expressed?
A hyperbolic curve, which is expressed as a percent rather than as a fraction
What are the common unitary prefixes relevant to pharmacodynamics?
milli = 10-3
micro = 10-6
nano = 10-9
pico = 10-12
On a -log curve of drug concentrations, what point is most commonly used as a comparison between two drugs plotted on the same curve?
The halfway point (50% occupancy), also known as EC50
What are the three terms that relate to the ability of a drug to bind to it’s receptor? What axis is this conveyed on in a graph?
Potency
Affinity
KD
EC50 (50% of maximally effective conc. for an individual)
ED50 (same as EC50 but with “dose” for a population)
These are all conveyed on the x-axis, shifts left when it is more potent (less required to generate a response)
What are the three terms that indicate the ability of a drug to produce a response? What axis do these terms convey a response on?
Efficacy
Power
Intrinsic activity
These aspects of drug effectiveness determine where the drug “tops out” asymptotically on the y-axis–not all drugs have the same efficacy! Those that have lower asymptotes are not as effective.
How do agonists work?
Agonists activate receptors and create a response


