Synaptic Transmission and Neural Plasticity Flashcards
Axon Hillock
the beginning of the axon before the axon proper
What are the two types of synaptic transmission responses?
- Direct excitatory neurotransmission (direct)
- the membrane of the next cell becomes either depolarized or hyperpolarised
- Neuromodulation (indirect)
- alters the presynaptic cells ability to produce/release a neurotransmitter are alters the postsynaptic cell’s ability to respond to the neurotransmitter
Criteria for a chemical to be a neurotransmitter
- synthesized in the neuron
- present in the presynaptic terminal and released in amounts sufficient to produce a defined effect on the postsynaptic neuron or effector organ
- when administered exogenously it mimics the action of the endogenously released transmitter
- a specific mechanism exists for removing it from the synaptic cleft
Synaptic Vesicles
- vesicles are anchored to the cytoskeleton by synapsin
- Ca2+ activates Calcium calmodulin activated kinase II (CaMKII) phosphorylated synapsin
- P-synapsin can no longer bind to the cytoskeleton, vesicles dock to the active zone
- voltage-gated Ca2+
- SNARE complex at the active zone
- SYnaptobrevin and Synaptogotagmin
go over
Cleavage of SNARE proteins by cordial toxins
- Botulinum toxin
- neuromuscular transmission ACh
- Tetanus Toxin
- interneurons at spinal cord, GABA Gly
Diseases that affects presynaptic terminal;
- Congential myasthenic syndromes: impaired vesicle recycling
- Latrotoxin: triggers vesicle fusion
- Botulinum and tetanus toxins: affect snare proteins involved in vesicle formation
- LEMS attack presynaptic Ca2+ channels
- Cognitive disorders: impair transsynaptic signalling
Synaptic membrane transporters
- Vesicular transporters powered by proton gradient
- use ATPase proton pumps
- make vesicles acidic (pH5.5)
- 1 glutamate for 1H+ (a counter-transport mechanism)
- Plasma membrane transporters powered by electrochemical gradient
- Na+ higher and K+ higher inside
- Glutamate co-transported with 2 Na+ molecules
Overview of the Categories of neurotransmitters
- Amino acids: faster (glutamate)
- Synthesized locally in presynaptic terminal
- Monoamines:
- Stored in synaptic vesicles
- Acetylcholine:
- Released in response to local increase in Ca2+
- Neuropeptides: slower
- Synthesized in the cell soma and transported to the terminal
- Stored in secretory granules
- Released in response to global increase in Ca2+
Explain the differential release of neuropeptides and small molecule co-transmitters
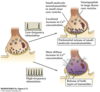
Fast Transmission Amino Acid transmitters
- Excitatory: slightly depolarises the postsynaptic cell’s membrane
- Glutamate (Glu) (CNS)
- 2) Inhibitory: slightly hyperpolarises the postsynaptic cell’s membrane
- (γ-aminobutyric acid) GABA (brain)
- Glycine (Gly) (spinal cord and brain stem)
Diffuse Modulatory System: Serotonergic system
Function in: mood, sleep, pain, emotion, appetite
- produced by a small set of neurons in the brain stem: Raphe nuclei
- produced in several areas in the brain
the Neuronal Layers of the brain
- 1-6/ A-G
- A- Pyrimdal neuorns
- B - Spiny Stellate neurons
- G - Chandelier
- Layers 3/4 have a lot of cortical input, but mainly from the thalamus (main periphery relay channel)
- Layers 5/6 mainly take projections towards the cortical structures: they feedback to thalamus with processing information for motor functions.
- Excitation: Glu
- Inhibition: GABA
Glutamate (Glu)
- Synthesied in presynatic terminals
- from glucose in the Krebs cylce
- from glutamine converted by glutaminase
- loaded and stored in vesicles by vesicular glutamate transporters (VGLUTs)
- reuptake by excitatory amino acid transporters (EAATs) in the plasma membrane of presynaptic cell and surrounding glia
- glial cells convert Glu to glutamine and this is transported from the glia back to nerve terminals where it is converted back into Glutamate.
GABA (γ-aminobutyric acid)
- synthesized from glutamate (Glu) in a reaction catalyzed by glutamic acid decarboxylase (GAD)
- loaded and stored into vesicles by a vesicular GABA transporter, GAT (Gly uses the same transporter)
- cleared from synapse by reuptake using transporters on glia and neurons including non-GABAergic neurons
- GABA is made de novo more often than it is recycled
When amino acid transmitter release is not regulated
Causes
- too much Glu/too little GABA: hyper-excitability| epilepsy| excitotoxicity
- too much GABA: sedation| Coma
-
Cerebral ischemia
- the metabolic events that retain the electrochemical gradient are abolished
- reversal of the Na+ / K+ gradient
- transporters release glutamate from cells by reverse operation
- excitotoxic cell death (Ca2+ -> enzymes -> digestion)
-
GHB γ-hydroxybutyrate (date rape drug)
- a GABA metabolite that can be converted back to GABA
- Increases amount of available GABA
- too much leads to unconsciousness and coma
Types of Monoamines
- Catecholamines
- Dopamine
- Epinephrine (adrenaline)
- Norepinephrine
- Indolamines
- Serotonin (5-Hydroxytryptamine, 5-HT)
Catecholamine synthesis
- Tyrosine -> L-dopa (can cross blood-brain barrier)–> Dopamine
- Levodopa is administered for treating Parkinson’s disease
- Dopamine -(DBH)-> Norepinpherine NE -(PNMT)-> Epinephrine
- DBH (Dopamine B-hydroxylase) only in synaptic vesicles, NE is the only transmitter synthesised within vesicles
Catecholamine storage
- loaded into vesicles by Vesicular monoamine transporters (VMATs)
Catecholamine release and reuptake
- in the cytoplasm as well
- released by Ca2+ dependent exocytosis
- binds and activates the receptor
- reuptake of catecholamines terminates the signal
- reuptake powered by electrochemical gradient: created by dopamine transporters (DATs) and Norepipherine transporters (NETs
- in the cytoplasm catecholamines are:
- reloaded back into vesicles
- enzymatically degraded by Monoamine oxidase (MAO)
- inactivated by Catechol-o-methyl-transferase (COMT)
Drugs that affect the release and reuptake of catecholamines
- Amphetamine: reverses transporter so pumps out more transmitter and blocks reuptake (DA & NE)
- Cocaine and Methylphenidate (Ritalin): block DA reuptake into terminals. More DA in synaptic cleft – extended action on the postsynaptic neuron.
- Selegiline: MAO inhibitor found in dopaminergic nerve terminals thus preventing the degradation of DA allowing more to be released on subsequent activations ( treatment of early-stage PD, depression and dementia).
- Entacapone: COMT inhibitor (treatment of PD), doesn’t allow DA to be broken down
What are these abbreviations:
NETs:
DATs:
MAOs:
COMT:
SERTs
- NETs: Norepinepherine transporters
- DATs: Dopamine transporter
- MAOs: Monoamine oxidases
- COMT: Catechol-o-methyl-transferase
- SERTs: Serotonin transporters
Serotonin Synthesis
- Tryptophan –> 5-Hydroxtryophan–> 5-Hydrotryptamine
Serotonin storage, release
- stored in vesicles
- signal terminated by reuptake by Serotonin transporters (SERTs) on presynaptic membrane
- destroyed by MAOs in the cytoplasm
Drugs that affect serotonin release and reuptake
- Fluoxetine (Prozac): blocks reuptake of serotonin (SSRI – selective serotonin reuptake inhibitor) (treatment of depression, OCD)
- Fenfluramine: stimulates the release of serotonin and inhibits its reuptake (has been used as an appetite suppressant in the treatment of obesity)
- MDMA: methylenedioxymethamphetamine (ecstasy) causes NE and serotonin transporters to run backwards releasing neurotransmitter into synapse/extracellular space (assessed for therapeutic potential in PTSD)









