Session 5 Flashcards
(51 cards)
What is Hypoxia?
[*] a fall in alveolar, thus arterial pO2. pO2 may vary over quite a range around the normal value of 13.3 kPA without any alteration in the degree of saturation of the pigment
Explain about Hypocapnia and Hypercapnia
[*] Hypercapnia: a rise in alveolar, and hence arterial pCO2
[*] Hypocapnia: a fall in alveolar, and hence arterial pCO2
- The alveolar pCO2 has a very important role as CO2 forms part of the principal system controlling acid base balance.
- The pH of hydrogen carbonate/dissolved CO2 solution is determined by the Henderson-Hesselbalch equation: pH = pK + log ([HCO3-]/(pCO2 x 0.23))
- pCO2 x 0.23 = [dissolved CO2] when pCO2 is in kPa
- [Dissolved CO2] is therefore determined by the partial pressure of CO2, as the solubility is fixed
- Crucially therefore the pH is determined by the ratio of [HCO3-] to pCO2 which is normally about 20:1 – not on their absolute values
- Changes in pCO2 therefore affect pH of arterial blood.
- Changes in pCO2 may be used to compensate for changes in pH consequent upon alteration of [HCO3-] (e.g. if metabolic acid is buffer). pCO2 therefore needs to be controlled to keep pH stable, but may itself be used to control pH changes brought about in other ways.
- The kidneys also control [HCO3-} by variable excretion and synthesis so they may compensate for persisting changes in pCO2 by altering [HCO3-]
Describe hypoventilation and hyperventilation
[*] Hypoventilation: removal of CO2 from lungs is less rapid than its production – the inability to normally ventilate the lung; ventilation decreases with no change in metabolism (breathing less than you actually have to)
[*] Hyperventilation: removal of CO2 from lungs is more rapid than its production; ventilation increases with no change in metabolism (breathing more than you actually have to)
NB: in exercise, ventilation increases because metabolism increases (not hyperventilation)
Describe the effects of plasma pH on hyper- and hypo- ventilation
[*] pCO2 affects plasma pH (Henderson-Hasselbach): pH = 6.1 + log ([HCO3-] / (pCO2 x 0.23))
[*] Hyperventilation: pCO2 decreases, (pO2 increases), pH increases
[*] Hypoventilation: pCO2 increases, pH decreases
Describe the general effects of acute hypo- and hyper-ventilation
[*] Acute hypoventilation
- Hypercapnia and Respiratory Acidosis
- pH falls below 7.0
- Enzymes become lethally denatured
[*] Acute hyperventilation
- Hypocapnia and Respiratory Alkalosis
- pH rises above 7.6
- Free calcium concentration falls enough to produce fatal tetany. Ca2+ is only soluble in acid, so when pH rises, Ca2+ cannot stay in the blood. Nerves become hyper-excitable
- Voluntary hyperventilation (panic attack) is very common – twitches, tremors, can be dangerous
Define the terms ‘Respiratory Acidosis’, ‘Respiratory Alkalosis’, ‘Compensated Respiratory Acidosis’ and ‘Compensated Respiratory Alkalosis’
[*] Respiratory Acidosis: hypoventilation (CO2 is produced more rapidly than it is removed by the lungs) can lead to alveolar pCO2 rising (hypercapnia) so [dissolved CO2] rises more than [HCO3-} producing a fall in plasma pH.
[*] Compensated Respiratory Acidosis: if respiratory acidosis persist, the kidneys respond to low pH by reducing excretion of HCO3- restoring the ratio [Dissolved CO2]/[HCO3-], and the pH, near to normal. This takes 2-3 days.
[*] Respiratory Alkalosis: hyperventilation (CO2 is removed from alveoli more rapidly than it is produced) can lead to alveolar pCO2 falling, disturbing the ratio of [Dissolved CO2] to [HCO3-] so plasma pH rises.
[*] Compensated Respiratory Alkalosis: If respiratory alkalosis persist, the kidneys respond to the high pH by excreting HCO3- so the ratio [Dissolved CO2]/[HCO3-] returns near to normal, and therefore pH is restored, but buffer base concentration is reduced. This takes 2-3 days
Define the terms ‘Metabolic Acidosis’, ‘Metabolic Alkalosis’, ‘Compensated Metabolic Acidosis’, ‘Compensated Metabolic Alkalosis’
[*] Metabolic Acidosis: metabolic production of acid by the tissues => hydrogen carbonate is displaced from plasma as the acid is buffered therefore the pH of blood falls. This is a reduction of buffer base – metabolic acidosis.
Acid normally reacts with HCO3- in capillaries => breathed out
[*] Compensated Metabolic Acidosis: the ratio [Dissolved CO2]/[HCO3-] may be restored near the normal by lowering pCO2 (increased ventilation by the lungs), correcting pH, but the depletion of buffer base remains, until corrected by the kidney
[*] Metabolic Alkalosis: metabolic production of hydrogen carbonate => hydrogen carbonate is retained in the plasma therefore the pH of blood rises e.g. after vomiting
[*] Compensated Metabolic Alkalosis: the ratio [Dissolved CO2/HCO3-} may be restored near the normal by increasing pCO2 (decreased ventilation by the lungs), correcting pH but the alkalosis (excess of buffer base) is corrected by the kidney
Decreased ventilation generates a problem itself as it is reducing our oxygen intake
Describe the acute effects of falling inspired pO2
- Alveolar pO2 may vary considerably without affecting oxygen transport, though if it falls a great deal serious problems occur. We do not need to control pO2 precisely but must keep it above 8kPa
- Inspired pO2 is an insignificant influence upon respiration until it has fallen significantly but only small increases in inspired pCO2 produce large increases in ventilation rate
- The falling arterial pO2 is detected by Peripheral Chemoreceptors located in the Carotid and Aortic bodies
- The carotid and aortic bodies are stimulated by a decrease in oxygen supply relative to their own oxygen usage which is small. They only respond to large drops in O2.
- Stimulation of the receptors:
- Increases the tidal volume and rate of respiration (increased breathing)
- Changes in circulation directing more blood to the brain and kidneys
- Increased pumping of blood by the heart (increased heart rate)
Describe the acute effects of increasing inspired pCO2
[*] We need to control pCO2 precisely to avoid acid base problems. The alveolar pCO2 markedly affects plasma pH and disturbances of pH arising from persisting alterations of pCO2 are slowly corrected by the kidneys (through reduced/increased excretion of HCO3-)
[*] Sometimes ventilation is changed to correct metabolic disturbances of pH.
[*] Changes in pH following metabolic production of acid or alkali may be corrected by alteration of alveolar pCO2
- The peripheral chemoreceptors in the carotid and aortic bodies also detect changes in pCO2 but are insensitive
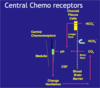
- Central chemoreceptors in the medulla of the brain are much more sensitive, altering breathing on a second to second basis.
- Central chemoreceptors detect changes in arterial pCO2
- Small rise in pCO2 would increase ventilation
- Small fall in pCO2 would decrease ventilation
- Are the basis of negative feedback control of breathing
Describe the acute effects of falling arterial plasma pH
- Metabolic Acidosis: metabolic production of acid displaces HCO3- from plasma as the acid is buffered; therefore the pH of blood falls.
- Compensated Metabolic Acidosis: the rate of [Dissolved CO2] to [HCO3-] may be restored to near normal by lowering pCO2 through increasing ventilation to correct pH.
Describe the location and function of the peripheral chemoreceptors and their role in the ventilator and other responses to Hypoxia
[*] The arterial pO2 must be detected by chemoreceptors. Receptors sensitive to falling pO2 are located in the carotid bodies and aortic bodies
[*] The carotid and aortic bodies are stimulated by a decrease in oxygen supply relative to their own oxygen usage which is small.
[*] A high rate of blood flow through the structures ensures that they do not normally change their response until the pO2 is low. They only respond to large drops in O2.
[*] Stimulation of the receptors has a variety of effects
- Increase in tidal volume and rate of respiration
- Changes in circulation directing more blood to brain and kidneys
- Increased pumping of blood by the heart
[*] They have slight tonic (continuous) activity and only respond to large falls in pO2 but they do not adapt.
[*] There is a respiratory drive as long as pO2 is low.

Describe the location and function of the central chemoreceptors
[*] Chemoreceptors sensitive to raised pCO2 are located both in the carotid and aortic bodies – the peripheral chemoreceptors, as well as in the medulla – the central chemoreceptors.
[*] Peripheral chemoreceptors are not particularly sensitive, needing a rise of 1.3kPA in pCO2 to stimulate them, whereas the whole animal responds to a rise of 0.3kPa. They are not crucial for the precise regulation of respiration but do respond QUICKLY to large changes in pCO2. Central Chemoreceptors are much more sensitive, altering breathing on a second to second basis
- Small rise in pCO2 leads to increased ventilation
- Small falls in pCO2 lead to decreased ventilation
[*] Central Chemoreceptors are located on the ventral surface of the medulla and exposed to the cerebro-spinal fluid. They respond to a fall in CSF pH.
Describe the central chemoreceptors’ role in the ventilatory respiratory to changes in arterial pCO2 and the roles of the cerebro-spinal fluid, blood brain barrier and choroid plexus in that response
- The CSF is separated from the blood by the Blood Brain Barrier, which allows free passage of CO2 but not HCO3- or H+.
- The pH of the CSF is determined by its own hydrogen carbonate / carbonic acid buffer system.
- No haemoglobin in CSF
- CSF [HCO3-] is determined by the activity of choroid plexus cells which pump HCO3- into and out of the CSF and is largely independent of plasma [HCO3-]
- CSF [Dissolved CO2] is determined by plasma pCO2 although rapid changes in plasma pCO2 take time to influence CSF [Dissolved CO2]
- Just as in plasma, therefore, CSF pH and thus the stimulus to the chemoreceptors is determined by the ratio of [HCo3-] to [Dissolved CO2]
- [*] If arterial pCO2 changes, then after a short delay, CSF pCO2 will follow. This leads to changes in CSF pH which are sensed by central chemoreceptors => normally producing changes in breathing which tend to restore CSF pH (i.e. if pCO2 rises, ventilation increases to lower pCO2 again). This negative feedback is the principal means by which ventilation is controlled.
What happens if this negative feedback doesn’t occur?
[*] If the negative feedback doesn’t occur e.g. due to an additional stimulus to ventilation from hypoxia, or because of disease of the lung, persisting changes in CSF pH stimulate the choroid plexus cells to pump more or less hydrogen carbonate into the CSF and change [HCO3-] to bring the ratio [HCO3-]/[Dissolved CO2] back towards normal.
Why is CSF pH corrected more quickly than blood pH?
[*] CSF is corrected much more quickly than blood pH because of its small volume. As CSF pH is corrected, changes in ventilation driven by alteration of pCO2 disappear and the control system is ‘reset’ to operate around a different pCO2.
- In the short term, [HCO3-} is fixed (cannot cross BBB) so falls in pCO2 lead to increased pH and rises in pCO2 lead to lower pH.
- If pCO2 remains altered for any length of time, the activity of the choroid plexus cells serves to ‘reset’ the system to control around a different pCO2: as long as CSF returns to normal, you stop feeling breathless – central chemoreceptors are happy. Over a slightly longer time scale, kidneys change [HCO3-] so you can live with higher plasma CO2 in the long term (but body does not tolerate short term variations)
- Long term changes: persisting hypercapnia, persisting hypoxia
Describe hypoxia (think about the oxygen transport chain, the carbon dioxide transport chain etc)
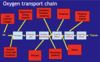
[*] The steps in the chain of oxygen supply: air => airways => alveolar gas => alveolar membrane => arterial blood => regional arteries => capillary blood => tissues
- The oxygen content of arterial blood depends on the pO2 and the Hb content of blood
- The delivery of oxygen to the tissues depends on the cardiac output and a normal vascular system permitting normal perfusion of tissues.
- The steps in the carbon dioxide transport chain: Tissues => Capillary blood => Region veins => Venous blood => Alveolar membrane => Alveolar gas => Airways => Air
Describe ventilation and gas exchange briefly
[*] Ventilation:
[*] NB: there are no exhaust valves so the values of pO2 and pCO2 are different in air and atmospheric air because of inspired and expired gases mixing in the same tube – ventilation is not 100% efficient.
[*] Gas exchange in normally 100% efficient so same values for alveolar air and arterial blood.
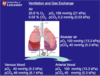
What could hypoxia be caused by?
- Hypoventilation
- Poor ventilation and perfusion matching (V/Q mismatch)
- Diffusion impairment
- Low inspired pO2: this occurs in people acutely exposed to high altitudes where the atmospheric pressure is less than 101 kPa as would occur if a passenger airplane cabin becomes depressurised or in acute mountain sickness. People who live at high altitudes have numerous physiological adaptations to survive in these conditions such as polycythaemia (increased Hb), increased red cell 2-3 DPG and increased capillary density in tissues
- Right to left shunts (revise CVS!!)
Describe Types 1 and 2 Respiratory Failure
[*] Respiratory failure is usually said to exist when the arterial pO2 falls below 8kPa (60 mmHg) when breathing air at sea level
- Not enough oxygen enters the blood
- Not enough CO2 leaves the blood
- Do not necessarily occur together
[*] Type 1 Respiratory failure: low pO2 (<8kPa) with a normal or low pCO2 (CO2 removal not compromised but not enough oxygen enters the blood)
- Breathlessness, exercise intolerance, central cyanosis (of the tongue, mucous membranes – in mouth)
- Daily tasks e.g. getting out of bed can be challenging, very disabling
- May not be able to walk very far
[*] Type 2 Respiratory failure: low pO2 (<8kPa) with a high pCo2 of > 6.7 kPa (50 mmHg) (not enough oxygen enters the blood, not enough CO2 leaves it)
What could cause hypoventilation?
[*] The muscles of respiration’s movement is involuntary and depends on impulses which originate in the respiratory centre in the brain stem, and travels via the spinal cord, spinal and peripheral nerves and the neuromuscular junction to reach these muscles.
[*] Causes of hypoventilation – ineffective respiratory effort:
- Respiratory centre depression; e.g. Head injury, drug overdose (narcotics – opioid analgesics – adverse drug reaction)
- Respiratory muscle weakness due to damage/disease of any part of nerve pathways from the respiratory centre to the muscles of respiration (e.g. brain stem / spinal cord/ intercostal nerves/ phrenic nerve / neuromuscular junction / muscle disease)
- Chest wall problems (mechanical problems) e.g. scoliosis /kyphosis, morbid obesity, rib fractures, pneumothroax
- Hard to ventilate lungs due to severe lung fibrosis or widespread severe airway obstruction (life threatening asthma, late stages of COPD)
What could cause ventilation/perfusion mismatch?
Ventilation /perfusion mismatch (Type 1 respiratory failure – pCO2 is low/normal)
- Reduced ventilation of some alveoli: lobar pneumonia
- Reduced perfusion of some alveoli: pulmonary embolism
[*] Reduced ventilation causes a drop in alveolar pO2 due to low ventilation (V): perfusion (Q) ratio (low V/Q ratio) of these alveoli. The blood draining these alveoli is poorly oxygenated, causing a low pO2 in the blood reaching the left side of the heart.
[*] Causes of reduced ventilation:
Pneumonia
Early stages of Acute Severe Asthma
Respiratory distress syndrome of newborn (collapse of some alveoli due to lack of surfactant)
[*] Reduced perfusion of some alveoli, usually due to pulmonary embolism, causes diversion of blood to other parts of the pulmonary circulation.
The extra blood flow (increased perfusion Q) is not matched by the ventilation (V) of these alveoli. The reduced V/Q ratio causes the alveolar pO2 to fall and the blood draining these alveoli is poorly oxygenated, causing a low pO2 in the blood reaching the left side of the heart.
Poor O2 uptake in some alveoli cannot be compensated by increased uptake in others (the other alveoli are already working at full saturation – 100% efficiency)
[*] In either situation, the drop in pO2 in areas with a low V/Q ratio cannot be compensated by extra oxygen uptake by blood at better ventilated alveoli with a high V/Q ratio, due to nature of the oxygen Hb dissociation curve which means that blood (Hb) becomes fully saturated with O2 at a pO2 of 13.3 kPa and cannot further increase its O2 content despite exposure to higher alveolar pO2 levels.
Poor O2 uptake in some alveoli cannot be compensated by increased uptake in others, whereas low pCO2 removal in some alveoli can be compensated by increased CO2 washout in other alveoli, as CO2 easily diffuses across alveolar capillary membrane and pCo2 removal is not limited. Hence ventilation-perfusion mismatch causes Type 1 Respiratory failure
What could cause diffusion impairment?
- Diffusion impairment (Type 1 respiratory failure – pCO2 is low/normal): O2 diffuses much less readily than CO2 so is always affected first
- Structural changes: lung fibrosis causing thickening of alveolar capillary membrane. Lung fibrosis could be due to fibrosing alveolitis, extrinsic allergic alveoltis, pneumoconiosis (disease of coal miners), asbestosis
- Increased path length: pulmonary oedema
- Total area for diffusion reduced: emphysema
- Normally involves most of the alveoli
- The barrier to diffusion between alveolar air and pulmonary capillary blood is normally very small (<1 micron).
- Diffusion may be impaired if the barrier is thicker (as in lung fibrosis) or the diffusion pathway lengthens as in pulmonary oedema where the extra layer fluid increases the distance across which gases have to diffuse.
- Diffusion may also be impaired if the total area available for diffusion is reduced (even though the barrier itself is normal) as happens in emphysema
- O2 diffuses much less readily than CO2 and so it is always affected more by any change to the diffusion barrier. Therefore diffusion impairment causes Type 1 Respiratory failure with hypoxia with a normal or low pCO2.

Describe the normal ventilation-perfusion ratio in the lung
- Ideal ratio should be 1
- At the apex of the lung, ratio is 3.3 which indicates ventilation is too much for perfusion - blood flow is low partly due to upright posture (effect of gravity)
- For most of the lung, ideal ratio is 1

Describe measurement of oxygen saturation and blood gas analaysis
[*] Oxygen Saturation of haemoglobin in arterial blood (SaO2) can be measured by a pulse oximeter
- Non-invasive
- Device uses infrared light to measure amount of oxygenated haemoglobin
- SaO2 > 95% (97% is normal)
- Very useful for monitoring patients
[*] Blood Gas Analysis
- Arterial blood sample obtained by arterial stab radial artery usually used)
- Sample put through a blood gas analyser
- Blood has to be heparinised or treated with some other anti-clotting agent to prevent blood clotting
- Blood must be sealed properly to prevent gas mixing with atmosphere.
- Normally stored in ice cold water to limit gas diffusion