Session 11: Hypersensitivity Reactions Flashcards
(30 cards)
What do clinical immunologists do?
Immune mechanisms are involved in the pathogenesis of a wide range of diseases. Measurement of specific immune parameters can be of considerable benefit in the diagnosis and management of many conditions and may lead to the identification of new treatments for disease (e.g. anti-TNF therapies for severe cases of rheumatoid arthritis).
Clinical immunologists provide both laboratory services to aid in the diagnosis of immunological disorders, and clinics in which patients with immunological disorders are seen. Specialised laboratories are available in most centres, supervised by Clinical Immunologists, to test for these disease-associated immune responses, and a new area of clinical practice has grown out of the understanding of the important of immune mechanisms in the pathogenesis of disease.
Broadly, the diseases managed by clinical immunologists can be divided into three main groups of disorders: immunodeficiency (underactive immunity), allergy (aberrant immune to extrinsic antigens) and autoimmunity (aberrant immunity to intrinsic antigens). Immune mechanisms are involved in diseases affecting almost all organ systems of the body, and so clinical immunologists often work in association with organ specialists (e.g. respiratory physicians, renal physicians) in managing patients with complex, multisystem disorders.
What are hypersensitivity reactions? What are the two phases involved?
Hypersensitivity reactions: in some cases, excessive or overzealous immune responses can lead to tissue damage. These responses are known as hypersensitivity reactions. Hypersensitivity reactions form the basis of autoimmune diseases. However, hypersensitivity reactions can also occur in response to infectious gents (e.g. fulminant hepatitis in hepatitis B infection) or to environmental substances (e.g. hay fever or certain drug reactions). Common features include
- Sensitization phase: first encounter with the antigen (clinically silent, no clinical manifestations at this stage).
- Effector phase: clinical pathology upon re-exposure to the same antigen.
Hypersensitivity definition: the antigen-specific immune responses that are either inappropriate or excessive and result in harm to host.
The mechanisms underlying these aberrant immune responses are those employed by the host to fight infections.
What are the types of triggers?
Types of triggers (antigens)
Hypersensitivity to extrinsic antigens
- Non infectious substances (innocuous) e.g. pollen, peanuts
- Infectious microbes (e.g. gram negative bacteria can drive hypersensitivity => sepsis shock)
- Drugs (penicillin allergy can lead to anaphylaxis)
Hypersensitivity to intrinsic antigens
- Infectious microbes (mimicry) – structural component of the host protein
- Self antigens (autoimmunity)
Infections driving hypersensitivity reactions against self-antigens - examples include Rheumatic Heart Disease, Guillain-Barre Syndrome and Type 1 Diabetes. Hypersensitivity to self antigens leads to autoimmune diseases.
Rheumatic heart disease
- Streptococcus pyogenes
- Similar antigen in cardiac muscle => endocarditis.
- No longer a clinical problem because of antibiotics.
Guillain-Barre syndrome
- Campylobacter jejuni
- Immune system mounts a response. Myelin-associated gangliosides have similar protein to Campylobacter.
- Normally self-limiting
Type 1 Diabetes
- Coxsakieviruses (A2, A5 and A9)
- Pancreatic islet cells => deficiency of insulin (diabetes) + pancreatic necrosis
- (the immune system response attacks the pancreatic cells as well as the coxsakieviruses)
Four major types of hypersensitivity reaction have been defined, to classify the main immunological mechanisms involved in hypersensitivity reactions (the Gell and Coombs classification). Describe Type I
Type I or immediate (<30 minutes)
Responsible for allergies
IgE mediated
Environmental non-infectious antigens
Describe Type II
Type II or antibody mediated (5-12 hours)
The antigens recognised in this way may either be intrinsic (self) or extrinsic (adsorbed onto the cells during exposure to some foreign antigen, possibly as part of infection with a pathogen).
These antigens are recognised by macrophages or dendritic cells, which act as antigen-presenting cells => B cell response wherein antibodies are produced against the foreign antigen.
E.g. ABO blood incompatibility where the red blood cells have different antigens; B cell proliferation will take place and antibodies to the foreign blood type are produced. IgG and IgM antibodies bind to these antigens to form complexes that activate the classical pathway of complement activation to eliminate cells presenting foreign antigens. That is, mediators of acute inflammation are generated at the site and membrane attack complexes causes cell lysis and death. Response takes hours to a day.
Describe Type III
Type III or immune complexes mediated (3-8 hours)
Antigen-antibody complexes that are not adequately cleared by innate immune cells accumulate, giving rise to an inflammatory response and attraction of leukocytes.
These immune complexes insert themselves into small blood vessels, join and glomeruli causing symptoms. The depositions induce an inflammatory response and as a result of the action of cleaved complement C3a and C5a, which respectively mediate the induction of granule release from mast cells (from which histamine can cause urticarial), and recruitment of inflammatory cells into the tissue (mainly those with lysosomal action, leading to tissue damage through frustrated phagocytosis by neutrophils and macrophages). Opsonisation also occurs.
IgG and IgM mediated
E.g. SLE – nuclear antigens leads to effects such as nephritis, skin lesions and arthritis; RA
Describe Type IV
Type IV or cell mediated (24-48 hours)
Environmental infectious agents and self antigens
CD4+ helper T cells recognise antigen in a complex with class II major histocompatibility complex. The antigen-presenting cells in this case are macrophages that secrete IL-12, which stimulates the proliferation of further CD4+ T helper cells. CD4+ T cells secrete IL-2 and interferon gamma further inducing the release of other cytokines thus mediating the immune response. Activated CD8+ T cells destroy target cells on contact, whereas activated macrophages produce hydrolytic enzymes and, on presentation with certain intracellular pathogens, transform into multinucleated giant cells.
Describe clinical examples of Type 1 Hypersensitivity
Clinical Examples of Type 1 Hypersensitivity disease (IgE-mediated allergic reactions)
- Immune reactant: IgE
- Antigen: soluble antigen
- Effector mechanism: mast-cell activation after antigen cross-links IgE antibodies on mast cells => mast cell degranulation
Systemic anaphylaxis
- Common allergens: drugs, serum, venoms, peanuts
- Route of entry: either directly or following oral absorption into the blood
- Response: oedema, increased vascular permeability, tracheal occlusion, circulatory collapse, death
Acute Urticaria (wheal and flare – mast cells activated in the epidermis)
- Common allergens: animal hair, insect bites, allergy testing
- Route of entry: through skin
- Response: local increase in blood flow and vascular permeability
Allergic rhinitis (hay fever)
- Common allergens: pollens (ragweed, timothy, birch), dust mite faeces
- Route: inhalation
- Response: oedema of nasal mucosa, irritation of nasal mucosa
Asthma
- Common allergens: Danders (cat), pollens, dust mite faeces
- Route: inhalation
- Response: bronchial constriction, increased mucus production, airway inflammation
Food allergy
- Common allergens: tree nuts, peanuts, shellfish, milk, eggs and fish
- Route of entry: oral
- Response: vomiting, diarrhoea, pruritis (itching), urticarial (hives), anaphylaxis (rarely)
What are the signs and symptoms of anaphylaxis?
Life threatening Airway and/or Breathing and/or Circulation problems – signs and symptoms of anaphylaxis
- Swelling of the conjunctiva
- Runny nose
- CNS: lightheadedness, loss of consciousness, confusion, headache, anxiety
- Swelling of the lips, tongue and/or throat
- Respiratory: shortness of breath, wheezes or stridor, hoarseness, pain with swallowing, cough
- Heart and vasculature: fast or slow heart rate, low blood pressure
- Skins: hives, itchiness, flushing
- GI: crampy, abdominal pain, diarrhoea, vomiting
- Pelvic pain
- Loss of bladder control
Describe the treatment of anaphylaxis
IM adrenaline
- Reverses peripheral vasodilation and reduces oedema and alleviates hypotension
- Reverses airway obstruction/bronchospasm
- Increases the force of myocardial contraction (B2 receptors on the heart)
- Inhibits mast cell activation
Do not delay treatment! Monitor pulse, blood pressure, ECG and pulse oximetry
Timesaver vs lifesaver – IM is best (compared to SC administration) – faster and high PK
Multiple doses may be required (so patients are advised to carry 2 epipens)
Proper use of epipen
Intramuscular injection can be complicated in that the depth of subcutaneous fat varies and may result in subcutaneous injection, or may be injected intravenously in error, or the wrong strength used.
Because of various expressions of alpha1 or B2 receptors, depending on the patient, administration of adrenaline may raise or lower BP, depending on whether or not the net increase or decrease in peripheral resistance can balance the positive inotropic and chronotropic effects of adrenaline on the heart.
Marked B2 activity can cause a fall in plasma potassium, an elevation in plasma glucose and enhanced finger tremor with additional bronchodilation and bronchoprotection.
What is meant by Type II hypersensitivity?
Antibody-dependent activation of complement (leading to cell death, cytokine chemotaxis and amplification of inflammatory response)
Antibody-dependent cell-mediated cytotoxicity
Antibody-mediated modulation of cellular function (change in tissue activity).
Immune reactant is IgG, some IgM
Antigen is either cell- and/or matrix-associated antigen or cell-surface receptor. Effector mechanism is complement and FcR+ cells (phagocytes, NK cells) or antibody alters signalling respectively (for the two types of antigens above)

Give examples of Type II hypersensitivity leading to change in function?
Graves’ disease (HLADR3-DR8)
- Anti-thyroid stimulating hormone receptor Ab
- Most common cause of hyperthyroidism (female/male 10:1)
Myasthenia gravis (HLADR3-A1-B8)
- Anti-acetylcholine receptor Ab (90%)
- Causal-relation unknown
- Skeletal muscle weakness, paralysis (female/male 2:1)
- Diagnostic test: tensilon test (give Acetylcholinesterase inhibitor => improved muscle function)
Pernicious anaemia (HLADR2-DR3-DR4)
- Anti-intrinsic factor Ab (auto-antibody) (75%)/anti-gastric parietal cells (90%)
- Vitamin B12 deficiency (paraesthesia, numbness, cognitive changes, visual disturbance)
- Diagnostic test: Schilling test – give patient radio-labelled B12 to determine if patient has pernicious anaemia and to ensure that impaired absorption of B12 (with or without intrinsic factor) is not occurring due to damage to the intestinal mucosa from the conditions of malabsorption arising from B12 and folate deficiency themselves.
Give examples of Type II hypersensitivity leading to tissue damage
Goodpasture’s syndrome
- Anti-glomerular basement membrane
- Glomerulonephritis => nephritic syndrome, kidney failure
- Lung haemorrhage
- Diagnostic test: IgG deposition plus enzyme-linked immunosorbent assay (ELISA) to detect antibodies against alpha3-chain of type IV collagen.
Haematological diseases
- Transfusion reactions (a-type mixed with b-type leads to haemolytic destruction of doner cells)
- Rhesus haemolytic anaemia (RhoGam)
- Rh+ father
- Rh-mother carrying her first Rh+ fetus. Rh antigens from the developing fetus can enter the mother’s blood during pregnancy.
- In response to the fetal Rh antigens, the mother will produce anti-Rh antibodies.
- If the woman becomes pregnant with another Rh+ fetus, her anti-Rh antibodies will cross the placenta and damage the fetal red blood cells.
- Treatment: give the mother IgG anti-RhD (preventative measure)
- Autoimmune haemolytic anaemia (AIHA)
- Idiopathic thrombocytopenic purpura

What could autoimmune haemolytic anaemia be due to? And describe how you would test for it? (the Direct Coombs Test?)
Can be idiopathic, infections (e.g. mycoplasma, Epstein-Barr virus), autoimmune disorders (e.g. SLE) and/or lymphoproliferative disorders (associated with some leukaemias)
Direct Coombs test (direct antiglobulin) – used to test for autoimmune haemolytic anaemia
- Blood sample from a patient with immune mediated haemolytic anaemia: antibodies are shown attached to antigens on RBC surface
- The patient’s washed RBCs are incubated with antihuman antibodies (Coombs reagent)
- RBCs agglutinate: antihuman antibodies form links between RBCs by binding to the human antibodies on the RBCs. This is a positive test – visual indication that antibodies (and/or complement proteins) are bound to the surface of RBCs.

Describe the Indirect Coombs Test. What is used to test for?
Indirect Coombs test (indirect antiglobulin test) – used in prenatal testing of pregnant women and in testing blood prior to a blood transfusion.
- Recipient’s serum is obtained, containing antibodies.
- Donor’s blood sample (health RBCs) is added to the tube with serum. The RBCs are thus of known antigenicity (with known reference values).
- Recipient’s Ig’s that target the donor’s red blood cells form antibody-antigen complexes.
- Anti-human Ig’s (Coombs antibodies) are added to the solution.
- Agglutination of red blood cells occurs because human Ig’s are attached to red blood cells – this is a positive result.

What is meant by Type III hypersensitivity?
Antibody-mediated hypersensitivity (IgG or IgM)
Soluble antigens:
- Exogenous or endogenous
- Circulating immune complexes (can deposit anywhere => multi-systemic)
- Non-organ specific
- Tissue damage due to deposition
- Complex size
- Duration of antigen exposure
- Host response
- Local tissues (joint, kidney, vessels)

Examples of Type III hypersensitivity include RA, glomerulonephritis and SLE. Describe RA
Anti-rheumatoid factor IgG (75%)
Articular and extra-articular features
Episodes of inflammation/remission (difficult to predict)
Poor prognosis factors
- <30 years old
- High titre of Rheumatoid Factor
- Female
- DR4 allele (although causal relationship unknown)
- Joint erosions
But very effective treatment these days such as Infliximab (TNF-alpha – very effective)
Describe Glomerulonephritis and SLE
Glomerulonephritis (infectious)
- Bacterial endocarditis
- Hepatitis B infection
Systemic lupus erythematous
- Most prevalent immune complexes disease
- Ratio female:male (9:1 ) – especially young females
- 40-60% patients present with cardiac, respiratory, renal, joint and neurological features.
- Repeated miscarriage
- Every patient is unique but most common symptoms include low-grade fever, photosensitivity, fatigue, loss appetitie, BUTTERFLY MALAR RASH on face, ulcers, muscles aches, arthritis in joints, pleural inflammation, pericardium inflammation, fingers and toes – poor circulation
- Diagnosis
- Criteria (11) set by American Rheumatism Association – include
- Malar rash, photosensitivity, kidney, arthritis and laboratory criteria include anti-nuclear antibodies (ANA), anti-dsDNA antibodies and haemolytic anaemia.
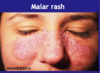
Describe Serology Tests
Sensitivity: % of patients with the condition that the test identified (eliminate false negatives)
Specificity: % of individuals that do not have the conditions that test excludes (eliminate false positives)
Ideally values should reach 100%. Diagnosis should be made taking into account clinical presentation and results of other investigations e.g. anti-ANA is a sensitive test for SLE however poorly specific, anti-dsDNA are detected in 80% of patients with SLE but specificity high (~100%)
Diagnosis should be based on various clinical and laboratory criteria.
What is meant by Type IV Hypersensitivity?
Type IV hypersensitivity: cell-mediated hypersensitivity reactions
Immune Reactant 1: TH1 cells. Soluble antigen => macrophage activation. Examples include contact dermatitis, tuberculin reaction.
Immune Reactant 2: TH2 cells. Soluble antigen => IgE production, eosinophil activation and mastocytosis. Examples include chronic asthma, chronic allergic rhinitis.
Immune Reactant 3: CTL (cytotoxic T lymphocyte). Cell-associated antigen => cytotoxicity. Examples include contact dermatitis such as nickel allergy, poison ivy.

Describe Tuberculin Hypersenitivity (Type IV)
Antigen is injected into subcutaneous tissue and processed by local antigen-presenting cells.
A TH1 effector cell recognizes antigen and releases cytokines, which act on vascular endothelium.
Recruitment of phagocytes and plasma to site of antigen injection causes visible lesion (reddish swelling)
Mantoux test: positive tuberculin test (48-72 hours) – screening test for latent TB.

Describe Granulomatous Hypersensitivity
Granulomatous Hypersensitivity – example of Type IV hypersensitivity
Granuloma: giant cells, T cells, necrosis => leads to lung damage
Goal of granuloma is to contain and seal off the infection
Other clinical conditions associated with granuloma formation
- Tuberculosis
- Leprosy (tuberculoid)
- Schistosomiasis
- Sarcoidosis (reason for development unknown)

Give examples of Type IV hypersensitivity autoimmune diseases including Coeliac disease
Insulin-dependent diabetes mellitus (islet cells)
Hashimoto’s thyroiditis (Thyroid peroxidase)
Rheumatoid arthritis (IgG)
Coeliac disease:
- Gluten-sensitive enteropathy
- Serology: anti-reticulin Antibody - sensitivity (90%)/specificity (>97%); anti-endomysial and TTG antibodies (100%) and (97%)
- Trigger: Gluten (breads, cakes, biscuit)
- Complications:
- Hyposplenism (Howell-Jolly bodies – basophilic nuclear remnants – clusters of DNA – in circulating erythrocytes)
- Lymphoma (50 fold higher risk)
- Jejunal biopsy: flattened mucosal surface, villous atrophy, mononuclear cell infiltrate