Session 1: Acid-Base Balance Flashcards
State the normal range of plasma pH
Normal cellular function depends critically on extracellular pH, which must be maintained within very narrow limits. This is achieved by an interaction between the respiratory and renal systems controlling different components of the buffer systems, which stabilise extracellular pH.
The normal range of plasma pH is 7.35-7.45 which is a concentration of between 34.5-44.5 nmol.l^-1 of H+. Both acidaemia and alkalaemia are very dangerous, with alkalaemia more so.

Describe the clinical effects of acidaemia and alkalaemia
Alkalaemia reduces the solubility of calcium salts, which means that free Ca2+ leaves the extracellular fluid, binding to bone and proteins. The resulting hypocalcaemia makes nerves much more excitable, producing paraesthesia and eventually uncontrolled muscle contractions – tetany. If the pH rises above 7.55, 45% of patients die, above 7.65 the mortality is 80%.
Acidaemia affects enzyme function in many tissues, and leads to K+ movement out of cells, producing a hyperkalaemia that can be fatal. Hyperkalaemia effects excitability, particularly cardiac muscle and lead to arrhythmia and even cause the heart to stop beating. Many enzyme functions are affects due to the increased [H+] leading to denatured proteins. This affects muscle contractility, glycolysis and hepatic function. The effects are severe at pH’s below 7.1 and life-threatening below 7.0.
Describe the carbon dioxide/hydrogen carbonate buffer system and the factors influencing pCO2 and [HCO3-]
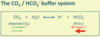
This is the most important extracellular buffer system: CO2 + H2O <=> H+ + HCO3-. The extent to which the reversible reaction proceeds is determined by the ratio of [dissolved CO2], itself determined by the pCO2 of plasma, which is controlled by the lungs, to [HCO3-], which is largely created by reactions in the red cell, but whose concentration is controlled by the kidney.
- pH = 6.1 + log ([HCO3-]/(0.23 x pCO2)).
- pCO2 is expressed in kPA and [HCO3-] in mmol.l^-1.
- Normal pCO2 is between 4.8 and 6.1 kPa – normal arterial pCO2 is 5.3 kPa.
- At pCO2 of 5.3kPa, water dissolves 1.2mmol.l^-1 CO2. Dissolved CO2 reacts with water in plasma and in cells to produce HCO3- and H+.
- Normal concentration of HCO3- in plasma is 22-26 mmol.l^-1.
- Should the pCO2 change, pH will be disturbed. Rises in pCO2 produce respiratory acidaemia (acidosis); falls in pCO2 produces respiratory alkalemia (alkalosis).
Describe the characteristics of respiratory acidosis & alkalosis and metabolic acidosis & alkalosis
- Respiratory acidosis: hypoventilation => hyerpcapnia => fall in plasma pH; characterised by high pCO2, normal HCO3- and low pH
- Compensated respiratory acidosis: high pCO2, raised [HCO3-] (by kidney) and relatively normal pH.
- Respiratory alkalosis: hyperventilation => hypocapnia => rise in plasma pH; characterised by low pCO2, normal HCO3- and raised pH
- Compensated respiratory alkalosis: low pCO2, lowered [HCO3-], relatively normal pH.
- Metabolic acidosis: initially characterised by normal pCO2, low HCO3- and low pH. Anion gap is increased if HCO3- is replaced by organic anion from an acid but normal if HCO3- is replaced by Cl-.
- Compensated metabolic acidosis: peripheral chemoreceptor (carotid bodies) detect pH drop (stimulate ventilation, leading to decreased pCO2) and it is characterised by lowered pCO2, low HCO3- and nearer normal pH.
- Metabolic alkalosis: normal pCO2, raised [HCO3-] and increased pH. Cannot be fully compensated – only partially compensated by reducing breathing due to the need to maintain pO2.
General Tips
If pCO2, is not normal, [HCO3-] is normal and pH has changed in opposite direction to pCO2 – this is respiratory acidosis/alkalosis
If [HCO3-] is not normal, pCO2 is normal and pH has changed in the same direction as [HCO3-] – metabolic acidosis/alkalosis
If pCO2 is high, [HCO3-] is raised and pH is relatively normal, it can only be compensated respiratory acidosis as metabolic alkalosis can’t be compensated.
If [HCO3-] is low, pCO2 is low and pH is relatively normal, it could be either compensated respiratory alkalosis or compensated metabolic acidosis. If no respiratory disease or altitude exposure, unlikely to be respiratory. If the anion gap is increased, it is compensated metabolic acidosis.
Describe the respiratory mechanisms controlling pCO2
Chemoreceptors normally operate to keep pCO2 stable. CO2 diffuses into the cerebrospinal fluid, changing its pH, which is sensed by the central chemoreceptors, which alter breathing to bring the pH back to normal. This system operates quickly and normally corrects respiratory pH disturbances, but they can persist in disease.

Describe the respiratory and kidney compensatory mechanisms
As the pH depends on the ratio of [HCO3-} to pCO2, not absolute values, respiratory acidaemia or alkalaemia can then be compensated by changes in [HCO3-] produced by the kidney. If pCO2 rises, a proportionate rise in [HCO3-] will restore pH, if pCO2 falls similarly a proportionate fall in [HCO3-] will restore pH. These compensatory changes are produced by variable renal excretion or production of HCO3-. But this takes time – 2-3 days.
If H+ ions are produced metabolically (e.g. from metabolism of amino aicds or production of ketones), they react with HCO3- to produce CO2 in venous blood, which is then breathed out through the lungs, leaving a directly proportional deficit of [HCO3-] in arterial blood (i.e. 1 mmol acid added to blood will remove 1 mmol HCO3-). Reductions in [HCO3-] are known as metabolic acidosis, increases are known as metabolic alkalosis.
These changes may be compensated by altering pCO2. In acidosis, pCO2 is lowered proportionately by increasing ventilation, in alkalosis, it may be slightly raised, but compensation is limited by hypoxia resulting from hypoventilation. These responses are mediated by the peripheral chemoreceptors in the carotid and aortic bodies.
How is HCO3- produced?
Hydrogen carbonate in plasma is not derived from CO2 in plasma – there is not enough CO2. It is made in red blood cells and transported to plasma. It is largely present in plasma and ECF as Na+ + HCO3- (dissociated sodium hydrogen carbonate).
The pH of arterial blood is determined by the ratio of pCO2 and [HCO3-] but although HCO3- in red blood cells, the [HCO3-] present in plasma is controlled by the kidneys.

Explain the cellular mechanisms of re-absorption of HCO3- in the proximal tubule
The handling of hydrogen carbonate by the kidney is therefore critical for maintaining acid-base balance.
Hydrogen carbonate is filtered in considerable quantities at the glomerulus – about 180 mmol.h^-1. This needs to be recovered to maintain acid-base status unless the patient is alkalotic. If the acid is produced metabolically recovery of all filtered HCO3- will be insufficient to restore plasma [HCO3-}, so [HCO3-] will have to be created within the kidney. This will create H+ which is then excreted directly or indirectly into the urine, and to avoid a damaging urinary acidity, it must be buffered by either other filtered substances or buffers created by the kidney.
Like most ions, a large fraction of HCO3- is re-absorbed in the proximal tubule. H+ ions are pumped out of the apical membrane of proximal tubule cells in exchange for the inward movement of Na+ down its concentration gradient. The reaction of H+ with HCO3- generates CO2 which enters the cell, and reacts with water to produce H+ which is exported, recreating HCO3- which leaves the cell to the plasma. 80-90% of the filtered HCO3- is re-absorbed in the proximal tubule. Up to 15% is also absorbed in the thick ascending limb of the loop of Henle by similar mechanisms.
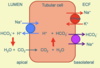
Explain the cellular mechanisms of H+ excretion in the distal tubule
The final site for HCO3- absorption and H+ excretion is the distal nephron (distal tubule and collecting ducts), through intercalated cells. Here, H+ is pumped across the apical membrane by a H+-ATPase pump (active secretion) as the Na+ gradient is insufficient to drive H+ out of the cell. By this stage there is very little HCO3- remaining, so little CO2 enters the cell to react with water and create H+ for excretion.
This is produced from CO2 produced by the cell’s own metabolism, so generating new HCO3- to enter the plasma. Most of the excreted H+ reacts with buffers (ammonia, phosphate) and remains in the urine.
Describe the mechanism of buffering of H+ in urine, and explain the concept of titratable acid, and the role of NH4+
Monobasic phosphate (HPO^2-,4) becomes more effective as the pH of the urine falls. The total buffering capacity of phosphate and the other weak acids is however limited by the amount filtered, and the replacement of any further HCO3- needs to take place by a mechanism within the kidney.
This is achieved by the excretion of ammonium ions (produced from glutamine): Glutamine => NH4+ + glutamate => NH4+ + alpha-ketoglutorate.
Each alpha-ketoglutorate yields 2 HCO3- which enters the bloodstream (in effect this is indirect excretion of H+ attached to ammonia). This process takes place largely in the proximal tubule, but is supplemented distally.
Excretion of ammonium is the major adaptive response to an increased acid load in healthy individuals.
Ammonium generation from glutamine in proximal tubule can be increased in response to low pH: NH4+ => NHS3 + H+
(NH3 freely moves into lumen and throughout interstitium; H+ actively pumped into lumen in DCT and CT; H+ combines with NHS3 => NH4+ (trapped in lumen)).
Overall, the kidney is able to both recover the filtered HCO3- and replace any which has been removed from the blood by the buffering of metabolic acid.
Describe the interactions between acid base status and plasma [K+]
Plasma potassium concentration also influences HCO3- re-absorption and ammonium excretion, so that as [K+] rises, the capacity of the kidney to reabsorb and create HCO3- is reduced. Hypokalaemia can therefore lead to metabolic alkalosis, and hyperkalaemia to metabolic acidosis.
Acidosis => hyperkalaemia: potassium ions move out of cells, decreased potassium excretion in the distal nephron
Alkalosis => hypokalaemia: potassium ions move into cells, enhanced excretion of potassium in distal nephron
Non-renal causes of metabolic acidosis cause increased re-absorption of K+ by kidneys and movement of K= out of cells => hyperkalaemia. In diabetic ketoacidosis, there may be a total body depletion of K+. K+ moves out of cells (due to acidosis and lack of insulin) but osmotic diuresis means K+ lost in urine. It can lead to hypokalaemia.
Describe the renal compensation in respiratory acidaemia/alkalosis
Acid secretion increases as extracellular pH falls, probably due largely to changes in renal tubular pH consequent upon changes in the rate of export of HCO3- to plasma, indicating that in reality it is [HCO3-] which is sensed and controlled rather more than pH per se. In respiratory acidaemia or alkalaemia, renal tubular pH is probably affected by changes in the rate of diffusion of CO2 in the cells as pCO2 alters.
In respiratory acidaemia, as pCO2 is increased, the ratio of HCO3-:pCO2 is decreased and so lowering pH. The fall in renal tubular pH induced by diffusion of extra CO2 into the cells leads to increased excretion of ammonium, with consequent production and export into the plasma of HCO3-, restoring the ratio of HCO3- to pCO2 nearer to normal.
In respiratory alkalaemia, rises in tubular pH induce less re-absorption of HCO3- by reducing H+ export and suppressing ammonium secretion – so HCO3- is excreted and [HCO3-] falls, again restoring the ratio of HCO3- to pCO2 nearer to normal.
When can metabolic alkalosis occur?
As the kidney filters huge amounts of HCO3- which have normally to be recovered, it should be easy to lose excess HCO3- by simply recovering slightly less. It is in fact possible to correct the consequences of huge infusions of HCO3- very quickly, so the accumulation of HCO3- alone in plasma will not produce a lasting alkalosis.
Metabolic alkalosis can occur, however, when other factors intervene to prevent increases in renal loss of HCO3-. The most common factor is body fluid volume contraction, leading to the need to recover sodium and potassium in large quantities from the filtrate, which leads to obligatory HCO3- recovery as well, which may even produce a paradoxical fall in urine pH.
Why does metabolic acidosis occur?
Metabolic acidosis will occur if there is excess metabolic production of acids (e.g. lactic acidosis, ketoacidosis), acids are ingested, HCO3- is lost, or there is a problem with renal excretion of acid.
If excess acid is produced, the associated anion (e.g. lactate, ketones or urate) will replace HCO3- in plasma, which will influence the anion gap. The anion gap is the difference between the sum of the measured concentrations of sodium and potassium and the sum of the measured concentrations of chloride and hydrogen carbonate. It is normally 10-18 mmol.l^-1. If hydrogen carbonate is replaced by another anion which is not included in the calculation the gap will increase. If the problem lies with the renal excretion of H+ this will change the [HCO3-] directly without replacement by an unmeasured ion, so the anion gap is less likely to change.
What conditions lead to respiratory acidosis?
Type 2 respiratory failure
[*] Low pO2 and high pCO2
[*] The alveoli cannot be properly ventilated
[*] COPD, severe asthma, drug overdose, neuromuscular disease (breathing is suppressed0
Can be compensated for by increase in [HCO3-].
Chronic conditions can be well compensated such that pH is near normal (by the kidney). Acute changes in CO2 can lead to acidosis (no time for kidney to compensate)
What conditions to respiratory alkalosis?
Hyperventilation
[*] Anxiety/panic attacks – acute setting
[*] Low pCO2, rise in pH
Hyperventilation in response to long-term hypoxia – Type 1 respiratory failure
[*] Low pCO2 with initial rise in pH
[*] Chronic hyperventilation can be compensated for by fall in [HCO3-].
[*] Kidneys can restore pH to near normal
What conditions lead to metabolic acidosis?
- If the anion gap is increased – indicates a metabolic production of an acid
[*] Keto-acidosis e.g. diabetes
[*] Lactic acidosis: e.g. exercising to exhaustion or due to poor tissue perfusion (e.g. in cardiogenic shock following MI)
[*] Uraemic acidosis: advanced renal failure – reduced acid secretion, build up of phosphate, sulphate, urate in blood.
- If the anion gap is normal, HCO3- is replaced by Cl-
[*] Renal tubular acidosis (RARE)
Problems with transport mechanisms in the tubules
Type 1 (distal) RTA – inability to pump out H+
Type 2 (proximal) RTA (very rare) – problems with HCO3- reabsorption
Can be hereditary or due to drug interactions
[*] Severe persistent diarrhoea can also lead to metabolic acidosis due to loss of HCO3- (replaced by Cl-)
What conditions lead to metabolic alkalosis?
In metabolic alkalosis HCO3- is retained in place of Cl-
Stomach is a major site of HCO3- production
[*] By-product of H+ secretion
[*] Severe prolonged vomiting – loss of H+
[*] Or mechanical drainage of stomach
Other causes:
[*] Potassium depletion/mineralocorticoid excess
[*] Certain diuretics (loop and thiazide)


