S3) Alcohol Metabolism & Oxidative Stress Flashcards
Where does alcohol metabolism occur?
- >90% alcohol is metabolised by liver
- Remainder is excreted passively in urine and on breath
What are the recommended limits for alcohol consumption?
14 units/week spread over at least 3 days for both men & women
Briefly describe the pathway involved in alcohol metabolism
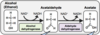
What happens when acetaldehyde accumulates?
- Acetaldehyde is a toxic metabolite
- Accumulation causes a “Hangover”
What happens to the acetate produced in alcohol metabolism?
- Acetate is conjugated to coenzyme A to form acetyl-CoA
- Acetyl-CoA is metabolised in TCA cycle / utilised for fatty acid synthesis
How is acetaldehyde toxicity controlled?
Acetaldehyde toxicity normally kept to a minimum by aldehyde dehydrogenase (low Km for acetaldehyde)
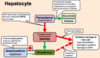
How does liver damage occur?
Prolonged and excessive alcohol consumption can cause sufficient acetaldehyde accumulation to cause liver damage
Identify three forms of liver damage resulting from prolonged and excessive alcohol consumption
- “Fatty liver”
- Alcoholic hepatitis
- Alcoholic cirrhosis
Indicate how liver damage can lead to changes in liver metabolism
- Excess NADH (decreased NAD:NADH)
- Excess Acetyl-CoA
What are the consequences of liver damage due to prolonged and excessive alcohol consumption?
- Lactic acidosis
- Fatty liver
- Hypoglycaemia
- Gout
Illustrate how excess NADH and Acetyl-CoA resulting from alcoholic liver damage can lead to the following consequences:
- Lactic acidosis
- Gout
- Hypoglycaemia
- Fatty liver

Which drug can be used to treat chronic alcohol dependence?
Disulfiram
Explain how Disulfiram treats chronic alcohol dependence
- Disulfiram is an inhibitor of aldehyde dehydrogenase
- If patient drinks alcohol acetaldehyde will accumulate causing symptoms of a ‘hangover’

Cellular damage caused by ROS & RNS is a significant component in a wide range of disease states.
Identify some
What is a free radical?
A free radical is an atom or molecule that contains 1/more unpaired electrons and is capable of independent existence e.g. OH•

Why are free radicals so damaging?
- Free radicals are usually very reactive and tend to acquire electrons from other atoms, molecules or ions
- Reaction of a radical with a molecule typically generates a second radical thereby propagating damage
What are the two types of free radicals found in the body?
- Reactive Oxygen Species
- Reactive Nitrogen Species
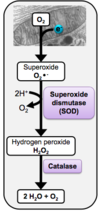
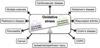
Describe the pathway involved in the formation of reactive oxygen species

Explain how reactive nitrogen species are formed
O2•- + NO• → ONOO-
- Superoxide can react with nitric oxide to produce peroxynitrite
- Peroxynitrite is not a free radical, but is a powerful oxidant which damages cells
Which three structures can ROS damage?
- DNA
- Proteins
- Lipids
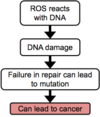
Outline the two ways in which ROS can damage DNA
- ROS reacts with base – modified base can lead to mispairing and mutation
- ROS reacts with sugar (ribose or deoxyribose) – causing strand break and mutation