Respiratory: Gaseous Exchange: Hypoxia and Hyper- and Hypo-Capnia Flashcards
Arterial Blood Gas Values
- Normal partial pressure for O2
- Normal partial pressure for CO2
- Hypoxaemia definition
- What is hypoxia?
- O2 = 13.3kPa
- CO2 = 5.3kPa
- Hypoxaemia is defined as an arterial PO2 below normal levels
- Tissue hypoxia describes when the PO2 within the cells is insufficient to allow normal aerobic metabolism to provide energy for cellular functions. This may occur despite a normal arterial PO2.
Causes of tissue hypoxia (4)
- Hypoxic hypoxia
- Anaemic hypoxia
- Ischaemic hypoxia
- Histotoxic hypoxia
Hypoxic hypoxia - is any cause of reduced oxygen availability to haemoglobin.
- Hypoxic atmosphere
- low O2 tension at high altitude
- Hypoventilation
- V/Q mismatch
- Shunt
- Deadspace
- Reduced O2 diffusion in the lung
- Loss of lung tissue e.g. emphysema
- Thickening of alveolar membrane e.g. pulmonary oedema
Anaemic hypoxia - is defined as any cause of reduced oxygen carrying capacity in the blood, for example:
- Reduced erythrocyte count
- Blood loss
- Marrow suppression
- Reduced haemoglobin concentration
- Iron deficiency
- Abnormal Hb
- Sickle cell
- Reduced Hb/O2 binding
- Carbon monoxide poisoning
Ischaemic hypoxia - also called circulatory or stagnant hypoxia, Hb and PO2 levels are normal, but tissue DO2 (oxygen delivery) is reduced. A greater proportion of O2 is extracted leading to an increase in arterio-venous O2 difference.
Causes include:
- Reduced cardiac output
- Hypovolaemia
- Primary cardiac failure
- Vascular abnormalities
- Embolism/external compression
- Arteriovenous shunting
Histotoxic hypoxia, oxygen delivery is maintained, but the cells are unable to utilize it, for instance in cyanide poisoning.
What is cellular metabolism
Three phases
Energy for cell functions comes from high energy compounds eg ATP
Simple (two carbon) units of metabolic fuels, such as carbohydrate, fat and protein are catabolized to produce these high energy compounds.
This process can be divided into three phases:
Phase 1: The production of two carbon compounds
Phase 2: The citric acid cycle
Phase 3: The electron transport chain
Cellular metabolism: Phase 1
Small components of metabolic fuels processed to produce 2 carbon compaounds for phase 2
Glucose
Glucose (6C) -> glycolysis -> 2 pyruvate (3C) (cytoplasm)
2 pyruvate (3C) -> oxidative carboxylation -> 2 acetylCoA (2C) + 2 CO2 (mitochondria)
Free fatty acids
Free fatty acids -> β oxidation -> AcetylCoA (mitochondria)
Amino acids
Amino acids -> oxidation -> pyruvate/AcetylCoA/Krebs cycle intermediates
Cellular metabolism: Phase 2
Krebs cycle (citric acid cycle)
AcetylCoA + oxaloacetate -> citrate -> krebs cycle
Each glucose produces 2x acetylCoA so 2x krebs cycle
Final compound is oxaloacetate, allowing the cycle to start again.
Per glucose molecule the cycle produces:
2 ATP
6NADH2+
2 FADH2
4 CO2
(reduced molecules containing high energy electons)
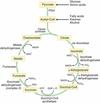
Cellular metabolism: Phase 3
The electron transport chain
Mitochondria
redcued enzymes re-oxidased
Releases
- electrons (passed down chain)
- energy -> converts ADP to ATP via oxidative phosphylation
NADH2+ releases energy to convert 3 molecules ADP
FADH2 releases energy to convert 2 molecules of ADP
Oxygen is the final electron acceptor -> H2O

Aerobic Respiration
All three phases proceed

Thirty eight molecules of ATP per glucose molecule
Anaerobic Respiration
electron transfer chain ceases to operate and oxidative phosphorylation stops
NAD+ and FAD are not re-formed, and so the Krebs cycle cannot continue.
Only glycolysis can continue to produce ATP by substrate phosphorylation.
From anaerobic glucose metabolism total energy production is therefore:
Two molecules of ATP per glucose molecule

ATP stores in the body will last?
What is the minimum PO2 for aerobic metabolism
Approx 90 seconds
Critical PO2 =0.4kPa
Consequences of cellular hypoxia

Draw the oxygen cascade

Physiological Compensation for Hypoxia
EARLY
Local
- Bohr effect
- right shift due to anaeorbic metabilsm producing acids -> decrease pH
- increase 2,3-DPG
- vasodilatation
- decrease pH, PO2,increase PCO2 and local metabolites (adenosine, K+)
Ventilatory
- peripheral chemoreceptors (carotid bodies) responding to fall in oxygen tension
- Hypoxic response
- PO2 <7 -> increase minute ventilation
- increase pCO2 -> increase minute ventilation
Cardiovascular
- perpheral chemoreceptors fall in O2 tension
- vasconstriction
- tachycardia
- -> increase CO and BP
LATE
- erythropoietin -> increase RBC (3-5 days)
- decrease O2 detected by renal peritubular interstitial cells
- 10% liver 90% kidneys

Hypoxia and the brain
Brain tissue relies entirely on oxidative phosphorylation of glucose for energy. Therefore it is extremely sensitive to hypoxia.
The brain utilises 20% of total body O2 consumption.
Cerebral blood flow (CBF) is maintained constant over a mean arterial pressure range of 50–150 mmHg.
Normally CBF is not affected by changes in PO2. A fall in PO2 below 6.7 kPa leads to exponential increases in CBF.
The increase in CBF is due to local lactic acidosis/vasodilatation.
Hypoxia and the heart
In coronary tissue, oxygen extraction is already very high at 75%, compared with 25% in other tissues.
Extraction can be increased as high as 90%, but if myocardial O2 need is high e.g. during exercise, or if hypoxia is present, O2 delivery must be maintained by increasing coronary blood flow.
Coronary blood flow may be increased by:
- Local metabolites causing arteriolar dilatation
- A direct effect of low O2 tension on arteriolar tone
- Myogenic control of arteriolar tone
Pulmonary response to hypoxia
Pulmonary tissue and hypoxic pulmonary vasoconstriction (HPV)
Unlike other systemic blood vessels pulmonary vessels constrict in response to a low PO2, principally in the alveolus but also in the pulmonary artery.
This response is locally mediated, and multifactorial. It may involve:
- Inhibition of nitric oxide production
- Local production of vasoconstrictors such as endothelin
- A direct effect of hypoxia on vascular smooth muscle tone
HPV results in the diversion of blood to the more oxygenated areas of the lung, improving oxygen uptake and delivery.
Normal ranges for PCO2
Arterial 4.7–5.3 kPa
Venous 6.1 kPa
Inspired PCO2 ≈ zero
Factors Affecting PaCO2
- Alveolar minute ventilation (AMV)
- Tissue metabolism (CO2 production)
The very rapid equilibration of CO2 across the alveolus means that PaCO2 is much less affected by changes in V/Q than PaO2.

Alveolar CO2
%alveolar PACO2= CO2 output x 100/VA
=200ml/min
4000ml/min
=5%
double VA = half PACO2
PaCO2 and Blood pH
Define pH
How are PCO2 and [H+] related
pH = - Log10 [H+]
By changing the PCO2 the equation proceeds to the left or the right, and a constant hydrogen ion (pH) concentration can be achieved.

Causes of Hypocapnia
Primary respiratory
- Hypoxia, caused by:
- Low atmospheric O2
- Congenital heart disease
- Pneumonia
- Anxiety
- Pain
All of the above cause an increase in the alveolar minute ventilation. As the rate of CO2 production is unchanged the result is a decrease in end tidal and arterial PCO2.
Compensatory
Compensatory hypocapnia occurs in response to a metabolic acidosis. The possible causes of a metabolic acidosis include:
Addition of exogenous acid, e.g. salicylate
↑endogenous acid production e.g. lactate, ketoacids
↓excretion of normal acid production, e.g. renal failure
↑loss of base, e.g. bicarbonate
There is a compensatory increase in A to attempt to maintain the plasma pH in the normal range by reducing PaC02.
Causes of Hypercapnia
Increased inspired CO2
- Increased inspired CO2 occurs principally during mechanical ventilation procedures, e.g.:
- Re-breathing
- Addition of exogenous CO2
Primary respiratory
- Primary respiratory causes of hypercapnia can generally be defined asany cause of a reduced VA:
- CNS depression
- Type 2 respiratory failure
- Restrictive lung disease
- Increase in respiratory dead space
Increased CO2 production
- In some circumstances, e.g. during anaesthesia and ventilation, increased CO2 production can occur without compensatory hyper-ventilation.
- Sepsis
- Malignant hyperthermia
Compensatory
- Compensatory causes are those that are secondary to metabolic alkalosis e.g.:
- Loss of H+ ions, e.g. vomiting
- Hypokalaemia → renal H+ excretion
- Addition of exogenous alkali, e.g. bicarbonate
The minute ventilation is reduced to increase the PaCO2 and correct the pH. This is however limited by the hypoxic effect.
If the hypoventilation is severe enough to cause hypoxia below the threshold of approximately 7 kPa, it will trigger an increase in ventilation.
Effects of Hypercapnia
Neurological
- Increased cerebral blood flow
- secondary to vasodilatation and increased mean arterial pressure
- CBF increases by approximately 7-15 ml/100g/min for each kPa increase in PCO2
- CBF tends to normalise after prolonged hypercapnia as intracerebral pH is corrected
- CBF tends to normalise after prolonged hypercapnia as intracerebral pH is corrected
- Secondary to vasodilatation
- Narcosis
- Occurs at PCO2> 12 kPa
- Autonomic effects
- Increased circulating catecholamines
Respiratory
- Control of breathing
- mediated by central chemo-receptors in medullar near respiratory centre
- CO2 rapidly crosses the BBB. In the CSF it dissolves + dissociates to release H+ ions, cannot cross the BBB, pH CSF falls
- Increasing PCO2 causes a linear increase in minute ventilation
- Pulmonary vasoconstriction
- Alveolar PCO2 > 7 kPa leads to increased pulmonary vascular resistance
- Oxygenation
- O2 dissociation curve shifts to right
Cardiovascular
- Direct effects
- Myocardial: changes in pH impair HR and myocardial contractility. (opposed by increase circulating catecholamines)
- Blood vessels: Acidosis causes direct arterial vasodilatation (except in the pulmonary vessels where it causes vasoconstriction)
- Arrhythmia. Acidosis affects intracellular potassium levels in the myocardial conducting tissue, predisposing to rhythm disturbances
- Catecholamine effects
- Myocardial:acidosis causes increase catecholamine release from the adrenal medulla. Increase HR = SV -> increased CO However, in more severe acidotic states the direct effects prevail
- Blood vessels. In milder acidotic states the circulating catecholamines cause vasoconstriction
- Arrhythmia. Increased circulating catecholamines cause tachycardia and tachydysrhythmias
Biochemical
- potassium leakage from the cells and an elevated serum potassium.
- shift from ionized to unionized calcium
Clinical Signs of Hypercapnia
Respiratory
- Tachypnoea. This will be present unless centrally mediated hypoventilation is the cause of the hypercapnia
- Dyspnoea. Is particularly present where there is a mechanical cause of the hypoventilation, such as airway obstruction
Cardiovascular
- Tachycardia and increased blood pressure result from catecholamine effects
- Reduced peripheral vascular resistance causes flushed skin and bounding pulses
Neurological
- At PaCO2 levels greater than 12 kPa concious level is depressed, ultimately leading to coma
- At lower levels of hypercapnia confusion, muscle twitching and hand flap may be present


