Rates, Kinetics, Equilibrium Flashcards
For the generic chemical reaction:
aA + bB ⇒ cC + dD
where a, b, c, and d represent the coefficient of reactants A, B, C, and D respectively, what is the rate at which the reaction will proceed?
For the generic chemical reaction
aA + bB ⇒ cC + dD
the rate expression is:
rate = kobs [A]x [B]y
where kobs is the rate constant, and x and y are the reaction orders of A and B, respectively.
How is the overall order of a chemical reaction calculated?
The overall order of a chemical reaction is the sum of the reaction orders of all the reactants.
Define:
The rate constant of a chemical reaction
A chemical reaction’s rate constant, kobs, is the rate at which the reaction proceeds when the concentration of all reactants is 1M or 1 atm.
Define:
A zeroth order reaction
A zeroth order reaction is one whose overall reaction order is zero, and is independent of reactant concentration.
A zeroth order reaction’s rate is constant, and can be given by:
Rate = kobs
If a chemical reaction is zeroth order in [A], with A being one of the reactants, how does the reaction rate vary if [A] doubles?
The reaction rate is unchanged.
If the reaction is zeroth order in [A], that means that the rate can be described as:
rate = kobs[A]<span>0</span> = kobs
Changing the value of [A] doesn’t change the reaction rate.
Define:
A first order reaction
A first order reaction is one whose overall reaction order is 1, and whose rate depends on the concentration of only one reactant, in a linear fashion.
If A is the reactant on which the reaction rate depends, the rate will be:
Rate = kobs[A]1
If a chemical reaction is first order in [A], how does the reaction rate vary if [A] doubles?
The reaction rate doubles.
First order in [A] means the rate law is:
rate = kobs[A]1
Let [A]orig = x.
Then rateorig = kobs * x.
If [A] doubles, then
ratenew = kobs (2x) = 2 * rateorig
Define:
A second order reaction
A second order reaction is one whose overall reaction order is 2, and whose rate either depends on one reactant to the second order, or two separate reactants to the first order.
If the reactants for the reaction are A and B, the rate will be one of:
Rate = kobs[A]2
or Rate = kobs [B]2
or Rate = kobs [A]1[B]1
If a chemical reaction is second order in [A], how does the reaction rate vary if [A] triples?
The reaction rate increases by a factor of 9.
Second order in [A] means the rate law is:
rate = kobs[A]2
Let [A]orig = x
Then rateorig = kobs * x2.
If [A] triples, then
ratenew = kobs (3x)2 = 9 * rateorig
If a chemical reaction is first order in both [A] and [B], how does the reaction rate vary if [A] triples, while [B] is reduced by half?
The reaction rate increases by a factor of 1.5.
If the reaction is first order in [A] and [B] means the rate law is:
rate = kobs[A]1 [B]1
Let [A]orig = x and [B]orig = y
then rateorig = kobs * x * y.
If [A] triples and [B] decreases by half, then
ratenew = kobs * (3x) * (½y)
= 1.5 rateorig
For a reaction with chemical A as reactant, and kinetic data given below, what is the reaction’s overall rate law?
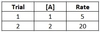
Rate = 5 [A]2
To determine the reaction order for A, see how varying [A] affects the rate. Between Trials 1 and 2, [A] increases by a factor of 2, and the rate increases by a factor of 4.
Since the rate increases by a factor of the concentration increase to the second power, the reaction must be second order in [A].
Once the reaction orders are solved, plug in the value of [A] for either trial to solve for kobs.
For a reaction with chemicals A and B as reactants, and kinetic data given below, what is the reaction’s overall rate law?
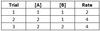
Rate = 2 [A]1 [B]0
Between Trials 1 and 2, [A] doubles, while [B] stays constant, and the reaction rate doubles, so the reaction order for A is 1.
Between Trials 2 and 3, [B] doubles, while [A] stays constant, and the reaction rate is unchanged, so the reaction order for B is 0.
Plugging in the values [A] = 1 and [B] = 1 from Trial 1 delivers a final value of 2 for kobs.
For a reaction with chemicals A and B as reactants, and kinetic data given below, what is the reaction’s overall rate law?
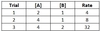
Rate = 2 [A]1 [B]2
Between Trials 1 and 2, [A] doubles, while [B] stays constant, and the reaction rate doubles, so the reaction order for A is 1.
Between Trials 2 and 3, [B] doubles, while [A] stays constant, and the reaction rate increases by a factor of 4, so the reaction order for B is 2.
Plugging in the values [A] = 2 and [B] = 1 from Trial 1 delivers a final value of 2 for kobs.
What is a reaction’s rate-determining step?
A chemical reaction’s rate-determining step is the slowest sub-step of a reaction. As such, it limits how fast the overall reaction can proceed.
Ex: the reaction
NO2 + CO ⇒ NO + CO2
is actually two sub-reactions,
1) NO2 + NO2 ⇒ NO3 + NO (slow)
2) NO3 + CO ⇒ NO2 + CO2 (fast)
The slow first step, is the rate-determining step, and limits the overall reaction rate.
How is a reaction’s rate-determining step identified?
The rate-determining step can be identified because the substances which appear in the rate law must be reactants in the rate-determining step.
Ex: If the overall reaction rate depends only on [NO2] for the following steps:
1) NO2 + NO2 ⇒ NO3 + NO
2) NO3 + CO ⇒ NO2 + CO2
Then the first step, in which NO2 appears as a reactant, must be the rate-determining step.
Define:
An intermediate in a chemical reaction
A reaction intermediate is a species that plays a role in a reaction, but does not appear in the overall chemical equation.
An intermediate can be identified by the fact that it is a product of an early reaction step, and a reactant in a later one.
Identify the intermediate in the reaction below:
Overall Reaction: 2 O3 ⇒ 3 O2
Step 1: O3⇒O2+ O
Step 2: O + O3⇒ 2 O2
O is the intermediate.
O is a product of Step 1, and a reactant of Step 2, and does not appear in the overall reaction. Therefore, it is an intermediate.
What is the molecularity of an elemental chemical reaction?
Molecularity is the number of reactant molecules taking part in a single reaction step. Therefore, it is a concept that can only be applied to elementary reactions.
A reaction involving one molecule of reactant is unimolecular; two is bimolecular; and three is termolecular.
What is the molecularity of the elementary reaction below?
NO + NO3→ 2NO2
NO + NO3→ 2NO2
The reaction has two individual molecules as reactants. Therefore, it is bimolecular.
In the chemical reaction Energy Profile shown below, where are the reactants and the products located?
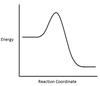
In Energy Profiles, the reactants are on the left, at the beginning of the reaction.
The products are on the right, at the end of the reaction.
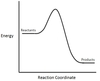
In the chemical reaction Energy Profile below, what is the quantity A?

A is the Activation Energy Ea.

The Activation Energy determines how much energy the reactants require to convert to the activated complex, or transition state. The higher the Activation Energy, the slower the reaction proceeds.
In the chemical reaction Energy Profile below, what portion of the reaction is located at point C?

The reaction’s Transition State is located at the peak of the reaction profile.
The Transition State, also known as the Activated Complex, is the chemical intermediate halfway between the reactants and the products. Since it is the highest energy point in the reaction profile, it is the least stable part of the reaction, and the reaction rapidly proceeds to the products once the transition state is reached.

In the chemical reaction Energy Profile below, what is the quantity B?

B is the Reaction Enthalpy, ΔH.

The Reaction Enthalpy is a measure of the difference in energy level between the reactants and the products.
If ΔH < 0, the reaction is exothermic.
If ΔH > 0, the reaction is endothermic.
Is the chemical reaction being depicted below endothermic or exothermic?

It is exothermic.

The products’ enthalpy is lower than the reactants’, so ΔH < 0, meaning the reaction is exothermic.




















