Organic Chemistry Flashcards
What is the IUPAC name of the following molecule?
2,3-dimethylpentane
1-t-butyl-2-methylbutane
2,3-dimethylhexane
2,2,3-trimethylpentane

2,2,3-trimethylpentane
What is the IUPAC name of the following molecule?
- 4-ethyl-5-methylhexane
- 3-isopropylhexane
- 3-ethyl-2-methylhexane
- 3-isopropylcyclohexane

3-ethyl-2-methylhexane
What is the IUPAC name of the following molecule?
- 2-hydroxy-2,7,7-trimethyloctane
- 2,7-trimethyl-7-octanol
- 2,7,7-trimethyl-2-octanol
- 2,7-trimethyl-2-octanol

2,7,7-trimethyl-2-octanol
What is the name of the following molecule?
3-ethyl-2,2,4,5-tetramethylheptane
3-tert-butyl-4,5-dimethylheptane
3,4-dimethyl-5-tert-butylheptane
3,5-diethyl-2,2,4-trimethylhexane

3-ethyl-2,2,4,5-tetramethylheptane
What is the IUPAC name of the following molecule?
7-ethoxy-2,3-dimethyloctane
2-ethoxy-6,7-dimethyloctane
2,3-dimethyl-7-ethoxyoctane
2-ethoxy-6-isopropylheptane

7-ethoxy-2,3-dimethyloctane
What is the name of the following molecule?
2-propyl-4-methylhexane
4,6-dimethyloctane
3,5-dimethyloctane
4,6-diethyloctane

3,5-dimethyloctane
What is the IUPAC name of the following molecule?
4-methyl-2-vinylpentane
2-methyl-3-pentyne
4-methyl-2-pentyne
4-methyl-3-pentyne

4-methyl-2-pentyne
What is the name of the following molecule?
phenylpropanoate
phenylethanoate
ethoxybenzenone
ethyl benzoate

ethyl benzoate
What is the IUPAC name of the following molecule?
1-butanenitrile
pentanenitrile
1-cyanobutane
1-butanamine

pentanenitrile
What is the IUPAC name of the following molecule?
3-ethyl-2-methylhexane
4-ethyl-5-methylhexane
3-isopropylhexane
3-isopropylcyclohexane

3-ethyl-2-methylhexane
What is the IUPAC name of the following molecule?
4-ethyl-5,7-dimethylnonane
5,7-dimethyl-4-ethylnonane
6-ethyl-3,5-dimethylnonane
2-ethyl-4-methyl-5-propylheptane

6-ethyl-3,5-dimethylnonane
What is the name of the following molecule?
2-methoxycyclopentanone
2-methoxy-1-pentanone
methylcylcopentanoate
methoxycylcopentanal

2-methoxycyclopentanone
What is the IUPAC name of the following molecule?
1,1-dimethyloctanamide
N,N-dimethylnonanamide
N,N-dimethylnonanamine
1,1-dimethylnonanamine

N,N-dimethylnonanamide
What is the IUPAC name of the following molecule?
cyclopentanamide
cyclopentanamine
1-aminopentane
1-aminocyclopentane

cyclopentanamine
What is the IUPAC name of the following molecule?
4-ethoxy-2-methylbutane
6-methyl-3-hexanone
1-ethoxy-3-methylbutane
ethyl isopentyl ether

1-ethoxy-3-methylbutane
What is the IUPAC name of the following molecule?
1-oxopentane
pentaldehyde
1-pentanone
pentanal

pentanal
What is the IUPAC name of the following molecule?
5-cycolpentylpentanoic acid
4-cyclopentylbutanoic acid
4-carboxybutylcycolpentane
carboxypropylcyclopentane

4-cyclopentylbutanoic acid
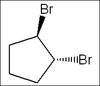
What is the name the following molecule?
1-methyl-2-ethyl-4-bromocyclohexane
1-bromo-3-ethyl-4-methylcyclohexane
5-bromo-2-ethyl-1-methylcyclohexane
4-bromo-1-ethyl-2-methylcyclohexane

4-bromo-1-ethyl-2-methylcyclohexane
What is the IUPAC name of the following molecule?
2,3-dimethyl-5-chlorohexane
4,5-dimethyl-2-chlorohexane
5-chloro-2,3-dimethylhexane
2-chloro-4,5-dimethylhexane

5-chloro-2,3-dimethylhexane
What is the IUPAC name of the following molecule?
7-hydroxy-3-heptanone
heptan-3-on-7-ol
3-keto-7-heptanol
5-oxo-1-heptanol

7-hydroxy-3-heptanone
What are the hybridization and bond angles respectively around the indicated atom?

sp2, 120
The indicated atom has 3 electron domains. According to VSEPR theory, the bond angles will be maximized and be ~120 . For 3 electron domains, the atom is sp2hybridized. Ultimately, with 3 electron domains it will need 3 hybrid orbitals and will need to mix 3 orbitals to make 3 hybrids. The 1st 3 orbitals available for making hybrids are an ‘s’ orbital and 2 ‘p’ orbitals, hence sp2.
How many sigma bonds are in the following molecule?

To be careful, you should draw out all of the bonds to the hydrogens that aren’t shown in the original structure.* There are 24 single bonds, 2 double bonds and 1 triple bond.* All single bonds are sigma bonds and the 1st bond of a double or triple bond is always a sigma bond.* The gives us 24+2+1=27

How many pi bonds are in the following molecule?

4
What are the hybridization and approximate bond angles respectively around the indicated atom?

sp3, ~109.5
The indicated atom has 4 electron domains.
According to VSEPR theory, the bond angles will be maximized and be ~109.5 (technically they’ll be a little less than 109.5 due to the repulsion of the non-bonding pair of electrons).
For 4 electron domains, the atom is sp3hybridized. Ultimately, with 4 electron domains it will need 4 hybrid orbitals and will need to mix 4 orbitals to make 4 hybrids. The 1st 4 orbitals available for making hybrids are an ‘s’ orbital and 3 ‘p’ orbitals, hence sp3.
Which of the following has the highest boiling point?
CH3CH2CH2F
CH3CH2CH3
CH3CH2CH2OH
CH3CH2CH2CH3
CH3CH2CH2OH Correct!
Which of the following has the highest vapor pressure?
CH3CH2CH3
CH3CH3
CH4
CH3CH2CH2F
CH4
The compound with the weakest intermolecular forces will have the highest vapor pressure. CH3CH2CH2F is the largest and only polar compound and therefore has the greatest intermolecular forces (both dipole-dipole and the greatest London Dispersion forces) and is eliminated. The remaining three choices are all nonpolar and have only London Dispersion forces but CH4 has the weakest as it is the smallest of the remaining answer choices and therefore has the highest vapor pressure.
Which of the following is most soluble in H2O?
CH3CH2CH2CH2CH2OH
CH3CH2CH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH
CH3CH2CH2CH2OH
HOCH2CH2CH2CH2OH Correct!
The governing principle in solubility is ‘like dissolves like.’ In this case H2O is very polar and capable of hydrogen bonding and the compound that is the most polar will be the most soluble in water. All of the answer choices are capable of hydrogen bonding having O-H bonds but choice C has 2 O-H bonds and is capable of twice as much hydrogen bonding and is therefore the most polar of the choices and is therefore the most soluble in water.
Which of the following has the highest boiling point?
CH3CH2CH2CH2CH2OH
CH3CH2CH2CH2OH
CH3CH2CH2CH2CH2CH2OH
HOCH2CH2CH2CH2CH2OH
HOCH2CH2CH2CH2CH2OH Correct!
The compound with the greatest intermolecular forces will have the highest boiling point. All of the answer choices have a hydroxyl group and are therefore capable of hydrogen bonding but only HOCH2CH2CH2CH2CH2OH has two hydroxyl groups and is therefore capable of a greater degree of hydrogen bonding and therefore has the greatest intermolecular forces and the highest boiling point.
Which of the following has the lowest boiling point?
CH3CH2CH2F
CH3CH2CH3
CH3CH2CH2OH
CH3CH2CH2CH3
CH3CH2CH3
The lowest boiling point will be for the compound with the lowest intermolecular forces. Choice C is capable of hydrogen bonding and choice A has dipole-dipole forces whereas choices B and D are non-polar and only have the relatively weak London dispersion forces. But choice B has a smaller surface area than choice D and therefore lower londong dispersion forces. It therefore has the lowest overall intermolecular forces of the choices listed and the lowest boiling point.
Which of the following has the highest boiling point?
CH3CH2OH
CH3OH
NaOCH3
CH3CH2Cl
NaOCH3
All of the answer choices listed are molecular compounds except for NaOCH3 which is ionic. To boil molecular compounds the intermolecular forces of the molecules in the liquid phase have to be overcome, but to boil an ionic compound ionic bonds have to be broken. Ionic bonds are typically much stronger than the intermolecular forces of molecular compounds and therefore ionic compounds tend to have much higher boiling points. This is the case here as NaOCH3 has the highest boiling point.
Which of the following is most soluble in benzene (C6H6)?
CH3CH2CH2OH
CH3CH2OH
HOCH2CH2OH
CH3CH2CH2CH2OH
CH3CH2CH2CH2OH Correct!
The governing principle in solubility is ‘like dissolves like.’ Benzene is non-polar and therefore whichever of the choices is least polar will be most soluble in benzene. In this case all of the choices are capable of hydrogen bonding making them at least somewhat polar. The key then is the compound with the largest non-polar region (in this case the longest carbon chain). The hydrocarbon portion of each molecule is non-polar and is largest in choice D. Therefore, choice D is the least polar and most soluble in benzene.
Which of the following will have the lowest vapor pressure?
CH3CH3
CH4
CH3CH2CH2CH3
CH3CH2CH3
CH3CH2CH2CH3
The compound with the greatest overall intermolecular forces will have the lowest vapor pressure. All four answer choices are nonpolar and therefore only exhibit London Dispersion Forces. CH3CH2CH2CH3 has the greatest weight and surface area and therefore has the greatest intermolecular forces and the lowest vapor pressure.
What does branching do to a boiling point and melting point?
You only use branching as a basis for comparing two isomers. More branching has higher melting point, but with more branching the boiling point is lower
What’s more stable a tertiary carbanion or a methyl carbanion?
methyl carbonion, because the problem with carbanions is that they have too many electrons so it doesn’t want anymore. With a tertiary carbanions, there would be 3 carbons donating some electron density
Which of the following is the strongest nucleophile in a protic solvent (H2O, CH3OH, etc.)?
OH-
SH-
F-
SeH-
SeH- Correct!
Protic solvents stabilize ions making them weaker nucleophiles but they stabilize small ions the most and therefore larger nucleophiles are stronger in protic solvents. According to the trend, SeH- is the strongest of the nucleophiles listed.
What’s the most stable kind of radical?
a tertiary one since it has 3 friends that donate electron density
A carbocation attached to a tertiary carbon who is attached to a benzene ring vs. a tertiary carbocation that is attached to the beneze ring? Most stable?
tertiary and benzylic and therefore also stabilized by resonance.
Which of the following is the strongest nucleophile?
F-
CH3-
OH-
NH2-
CH3- Correct!
The less the electronegative the atom, the stronger the nucleophile as it will be higher energy (less stable) and more willing to donate its electrons and therefore CH3- is the strongest nucleophile of the choices listed
Which of the following is the strongest nucleophile in an aprotic solvent (acetone, DMSO, etc.)?
SeH-
F-
SH-
OH-
OH- Correct!
Nucleophile strength increases as both size and electronegativity decrease in aprotic solvents. Therefore of the choices listed, OH-is the strongest nucleophile in an aprotic solvent
Which of the following statements is correct?
Molecules with resonance cannot be adequately drawn using a single Lewis structure
Resonance decreases the boiling point of compounds
Individual resonance contributors can be viewed using an electron microscope
Resonance causes molecules to be more reactive
Molecules with resonance cannot be adequately drawn using a single Lewis structure
How many degrees of unsaturation are present in C10H18?
4
5
2
3
2
A saturated hydrocarbon will have the generic formula CnH2n+2 and every two hydrogens less than this maximum equates to a degree of unsaturation. So 10 carbons could have a maximum of 22 hydrogens. Our compound, having only 18, is missing 4 hydrogens and therefore has 2 degrees of unsaturation.
How many degrees of unsaturation are present in C6H4Br2?
4
7
6
5
4
A saturated hydrocarbon will have the generic formula CnH2n+2 and every two hydrogens less than this maximum equates to a degree of unsaturation. The presence of halogens complicates this just a little. A halogen makes one bond just as hydrogen does and we therefore can count them as equal to a hydrogen for the purpose of comparing the “total” number of hydrogens in the compound to the saturated number. So this compound really has the equivalent of 6 hydrogens rather than 4 after counting the 2 bromine atoms.
So 6 carbons could have a maximum of 14 hydrogens. Our compound, having the equivalent of only 6, is missing 8 hydrogens and therefore has 4 degrees of unsaturation.
How can a reduction occur in an organic molecule?
Reduction can occur in one of three ways:
1) Addition of 2 bonds to hydrogen (usually the only atom less electronegative than carbon)
2) Loss of 2 bonds to atoms more electronegative than carbon (O, Cl, Br, etc.)
3) Addition of 1 bond to hydrogen and loss of 1 bond to an atom more electronegative than carbon
How can an oxidation occur in an organic molecule?
Oxidation can occur in one of three ways:
1) Addition of 2 bonds to atoms more electronegative than carbon (O, Cl, Br, etc.)
2) Loss of 2 bonds to hydrogen (usually the only atom less electronegative than carbon)
3) Addition of 1 bond to an atom more electronegative than carbon and loss of 1 bond to hydrogen
How many degrees of unsaturation are present in C4H10?
1
2
3
zero
zero Correct!
How many degrees of unsaturation are present in C6H7N?
7
6
5
4
8
A saturated hydrocarbon will have the generic formula CnH2n+2 and every two hydrogens less than this maximum equates to a degree of unsaturation. The presence of nitrogen complicates this just a little and can be accounted for in one of two ways.
1) Count the nitrogen has equal to half a carbon when calculating the saturated number of hydrogens. In this compound, we would therefore say we have 6.5 carbons which could have a maximum of 15 hydrogens. Our compound, having only 7, is missing 8 hydrogens and therefore has 4 degrees of unsaturation.
2) Use the normal formula (CnH2n+2) using just the number of carbons to determine the saturated number of hydrogens; for every nitrogen simply add another hydrogen to this total. So 6 carbons could have a maximum of 14 hydrogens but we’ll add another hydrogen to that total due to the presence of a single nitrogen. So the saturated number of hydrogens for this compound is actually 15. Our compound, having only 7, is missing 8 hydrogens and therefore has 4 degrees of unsaturation.
How many degrees of unsaturation are present in C4H6Cl2?
3
1
zero
2
1
A saturated hydrocarbon will have the generic formula CnH2n+2 and every two hydrogens less than this maximum equates to a degree of unsaturation. The presence of halogens complicates this just a little. A halogen makes one bond just as hydrogen does and we therefore can count them as equal to a hydrogen for the purpose of comparing the “total” number of hydrogens in the compound to the saturated number. So this compound really has the equivalent of 8 hydrogens rather than 6 after counting the 2 chlorine atoms.
So 4 carbons could have a maximum of 10 hydrogens. Our compound, having the equivalent of 8, is missing 2 hydrogens and therefore has one degree of unsaturation.
How many degrees of unsaturation are present in C6H12O?
3
zero
1
2
1
A saturated hydrocarbon will have the generic formula CnH2n+2 and every two hydrogens less than this maximum equates to a degree of unsaturation. The presence of oxygen doesn’t affect this at all and essentially can be ignored when calculating the number of degrees of unsaturation. So 6 carbons could have a maximum of 14 hydrogens. Our compound, having only 12, is missing 2 hydrogens and therefore has one degree of unsaturation.
How many degrees of unsaturation are present in C12H18N2?
10
5
16
20
6
5
How many degrees of unsaturation are present in C2H6O2?
3
zero
1
2
zero
How many degrees of unsaturation are present in C14H10?
9
7
8
10
10
Does high pKb correspond to a weak base or a strong base?
The base with the highest pKb is the weakest base. I
Charge – All choices have a negative charge.
Atom – The basic electrons are on 4 different atoms for the 4 bases and so this rule will determine the weakest base. The atom trend for basicity is below. The correct answer has the basic electrons on selenium which is the largest of the anions and therefore the weakest base.
Which of the following is the strongest base?
CH3S-
CH3O-
CH3Te-
CH3Se-
CH3O-
The basic electrons are on 4 different atoms (O, S, Se, and Te) for the 4 bases and so this rule will determine which is the strongest base. The atom trend for basicity is shown below. The basic atoms in each of these ions are all in the same group (column) on the periodic table. Base strength increases as you go up the periodic table as the smaller base forms a stronger bond with H+ and therefore CH3O- is the strongest base.
Which of the following is the strongest acid?
HF
H2O
NH3
CH4
HF Correct!
We rank acids by looking at the strengths of their conjugate bases (which are -CH3, -NH2, -OH, and F-respectively) knowing that the weakest conjugate base will have the strongest conjugate acid. I’ll use the CARDIO mnemonic for this one.
Charge – All choices have a negative charge.
Atom - The basic atoms in each of these ions (C, N, O, and F) are all in the same period (row) of the periodic table. Base strength increases as you go left on the periodic table (see diagram below) as in such an instance the less electronegative base is the less stable and stronger base. To find the strongest acid we’re looking for the acid with the weakest conjugate base which is F- as it is the most electronegative of the bases listed. Therefore HF is the strongest acid.
In the following molecule, which are the correct configurations (R and S) at each chiral center?

- S, 2.R, 3.S, 4.S Correct!
How are the following two compounds related?

same compound Correct!
Upon initial inspection these molecules are mirror images and you might be tempted to say they are enantiomers. But each has an internal mirror plane of symmetry and are therefore achiral (these are actually the same meso compound). And an achiral compound is identical to its mirror image by definition.
How many chiral centers are there in the molecule shown below?

4
chiral center has to be sp3 hybridized
How many stereoisomers will the following compound have?

16
2n = number of stereoisomers possible
When trans-2-butene is treated with Br2 and CH2Cl2, which of the following is true?
The product will not be optically active
2 new stereocenters are formed
The product is a meso compound
All of the above
all of the above
Arrange the following functional groups in order of priority (Highest*to lowest):
- -CH2C6H5
- –CH2CHO
- -SH
- -NO2
- -CH=CH2
3>4>5>2>1 Correct!
When propene is treated with Br2 and CH2Cl2, which of the following is true?
The product will be optically active
The product will be a racemic mixture
All of the above
2 new stereocenters are formed
The product will be a racemic mixture
Which of the following molecules has 3 equivalent staggered conformations?
BrCH2CH2Br
Br2CHCH2Br
BrCH2CBr3
Br2CHCHBr2
BrCH2CBr3 Correct!
The only way to have 3 equivalent staggered confirmations is for either the front or back carbons to have 3 identical substituents so that the 3 staggered confirmations are identical as in the choice C above.

Which of the following is an intermediate in the following reaction?




- O3 2. (CH3)2

- MCPBA 2. H3O+





Hg(OAc)2, H2O
What is the product of the following reaction?

NaNH2 is a really strong base and deprotonates acetylene forming the acetylide ion which is a good nucleophile. The acetylide ion then attacks CH3Br in an SN2 reaction. Another equivalent of NaNH2 then deprotonates the other sp-hybridized carbon forming another acetylide ion. This then attacks CH3CH2Br in another SN2 reaction leading to formation of the final product.

What is the product of the following reaction?

NaNH2 is a really strong base and deprotonates acetylene forming the acetylide ion which is a good nucleophile. The acetylide ion then attacks CH3Br in an SN2 reaction. Finally Sia2BH.THF followed by H2O2, OH-, H2O carries out hydroboration/oxidation which results in the anti-Markovnikov addition of water across the alkyne which initially forms an enol which tautomerizes to form an aldehyde as outlined below.

What is the product of the following reaction?

NaNH2 is a really strong base and deprotonates acetylene forming the acetylide ion which is a good nucleophile. The acetylide ion then attacks CH3CH2Br in an SN2 reaction. Finally H2SO4/H2O results in the Markovnikov addition of water across the alkyne which initially forms an enol which tautomerizes to form a ketone as outlined below.

What is the product of the following reaction?

NaNH2 is a really strong base and deprotonates acetylene forming the acetylide ion which is a good nucleophile. The acetylide ion attacks the carbonyl carbon, the electrophile, and that intermidiate is protonated by the hydronium to form an alcohol as shown in the mechanism below.

Which of the following is the correct set of reagents to carry out the following reaction?


What is the product of the following reaction?

Remember that a grignard reagent reacts just as if it were a carbanion, CH3- in this case, and is a good nucleophile. Here it attacks the carbonyl carbon, the electrophile, as shown in the mechanism below. The hydronium added in the 2nd step is there simply to protonate the intermediate forming an alcohol.

What is the product of the following reaction?

This one might be a little tricky as the last two steps don’t accomplish anything. The key is that we have an alkene at the end of step 3 and NaNH2 is only a strong enough base to deprotonate a terminal alkyne, but not a typical alkene or alkane. And since we don’t form an anion then in step four, then there isn’t a nucleophile to react with the propyl bromide in step 5.

What is the major product of the following reaction?

Br2/hv are the reagents for free radical bromination which substitutes a bromine for a hydrogen in the most stable position for a radical intermediate (3 >2 >1 >CH3). The carbon indicated below (red arrow) is the most substituted carbon that has a hydrogen explaining why choice A is the correct answer. Note that the carbon indicated by the black arrow is more substituted but doesn’t have a hydrogen to replace.

Which of the following would be the best set of reagents to carry out the following reaction?

The reaction is a free radical bromination and the only one of the reagents listed that corresponds to a free radical bromination is Br2/hv, the correct answer.
All of the other answer choices are reagents that undergo addition reactions with alkenes (or alkynes). As our reactant isn’t an alkene (or alkyne) they would all result in no reaction with the reactant alkane.

Which of the following would be the best set of reagents to carry out the following reaction?

The reaction is a free radical bromination at the allylic position. That is precisely when the best reagent to use is NBS/hv, the correct answer.
Br2/hv would also lead to some of the desired product but would also result in the addition of Br2 across the alkene thereby decreasing the yield of the desired product.
HBr and Br2/CH2Cl2 are both reagents that would undergo addition reactions across the alkene forming products other than the desired product.

How many monochlorination products (including all stereoisomers) could be obtained in the following reaction?

4
What is the equilibrium constant expression for the following reaction?
2Sb(s) + 3COCl2(g) → 2SbCl3(g) + 3CO(g)

Which of the following can result in a change in the value of an equilibrium constant?
A change in temperature
Adding an inert gas
A change in pressure
Increasing the concentration of reactants
A change in temperature Correct!
A change in temperature is the only thing that can change the value of an equilibrium constant. A change in pressure and an increase in the concentration of the reactants can result in a shift in the equilibrium according to Le Chatelier’s Principle but do not change the value fo the equilibrium constant.
If for the following reaction Kc = 107.
2Tl(s) + Co2+(aq) → 2Tl+(aq) + Co(s)
Then what is the value of the equilibrium constant for the following reaction?
4Tl(s) + 2Co2+(aq) → 4Tl+(aq) + 2Co(s)
428 9.3x10-3 1.1x104 214
1.1x104 Correct!
The reaction in question is related to the first reaction given in that all of the coefficients have been doubled. Doubling the coefficients leads to a squaring of the equilibrium constant which can be most easily seen by comparing the equilibrium constant expressions.

For the following reaction Kc = 2.2x10-9
HOBr(aq) → H+(aq) + BrO-(aq)
If [H+] = 2.2x10-5M, [BrO-] = 1x10-5M and [HOBr] = 0.1M then which of the following is true?
Not enough info is given to determine whether the reaction is at equilibrium or not
The reaction is at equilibrium
The reaction must proceed to the left to reach equilibrium
The reaction must proceed to the right to reach equilibrium
The reaction is at equilibrium Correct!

What is the equilibrium constant expression for the following reaction?
COCl2(g) + HCl(g) CHCl3(l) + ½O2(g)

If for the following reaction Kc = 1.7x107
Ag+(aq) + 2NH3(aq) → Ag(NH3)2+(aq)
Then what is the value of the equilibrium constant for the following reaction?
3Ag+(aq) + 6NH3(aq) → 3Ag(NH3)2+(aq)
- 7x106
- 7x1021
- 1x107
- 9x1021
4.9x1021 Correct!

The reaction in question is related to the first reaction given in that all of the coefficients have been tripled. Tripling the coefficients leads to a cubing of the equilibrium constant which can be most easily seen by comparing the equilibrium constant expressions.
For the following reaction Kc = 107.
2Tl(s) + Co2+(aq) → 2Tl+(aq) + Co(s)
If [Co2+] = 0.01M and [Tl+] = 2.0M then which of the following is true?
The reaction must proceed to the right to reach equilibrium
Not enough info is given to determine whether the reaction is at equilibrium or not
The reaction must proceed to the left to reach equilibrium
The reaction is at equilibrium
So K < Q and the reaction needs to proceed to the left (shift left) to reach equilibrium. Recall that when you write K before Q then the greater than or less than sign points in the direction the reaction needs to shift in order to reach equilibrium.

For the following reaction Kc = 1.7x107
Ag+(aq) + 2NH3(aq) → Ag(NH3)2+(aq)
If [Ag+] = 1x10-3M, [NH3] = 3x10-2M and [Ag(NH3)2+] = 0.9M then which of the following is true?
The reaction must proceed to the right to reach equilibrium
The reaction must proceed to the left to reach equilibrium
The reaction is at equilibrium
Not enough info is given to determine whether the reaction is at equilibrium or not
The reaction must proceed to the right to reach equilibrium Correct!
So K > Q and the reaction needs to proceed to the right (shift right) to reach equilibrium. Recall that when you write K before Q then the greater than or less than sign points in the direction the reaction needs to shift in order to reach equilibrium.

What is the equilibrium constant expression for the following reaction?
Fe(s) + CO2(g) → FeO(s) + CO(g)

If for the following reaction Kc = 2.0x10-9
HOBr(aq) → H+(aq) + BrO-(aq)
Then what is the value of the equilibrium constant for the following reaction?
H+(aq) + BrO-(aq) → HOBr(aq)
- 0x109
- 2.0x10-9 - 0x108
- 0x108
5.0x108 Correct!
The reaction in question is related to the first reaction given in that has been reversed. The equilibrium constant of a reverse reaction is equal to the inverse of the original reaction’s equilibrium constant which can be most easily seen by comparing the equilibrium constant expressions.
Which of the following changes to the following reaction at equilibrium would result in a shift to the right?
2Tl(s) + Co2+(aq) 2Tl+(aq) + Co(s)
removal of Co2+
addition of Tl+
addition of Tl
removal of Tl+
Removal of Tl+ Correct!
Generally the addition of a reactant or the removal of a product to a system at equilibrium will result in a shift to the right. But don’t forget that as solids and liquids don’t show up in equilibrium constant expressions, they therefore can’t result in a shift in the equilibrium. Therefore the addition of Tl won’t result in a shift at all and can be eliminated.
Of the three remaining answer choices only the removal of Tl+ involves either the addition of a reactant or the removal of a product and is the correct answer.
The addition of Tl+ is the addition of a product, and the removal of Co2+ is the removal of a reactant, both of which would result in a shift to the left and are therefore eliminated.
What is the equilibrium constant expression for the following reaction?
CaO(s) + CO2(g) → CaCO3(s)

What is the equilibrium constant expression for the following reaction?
2MnO4-(aq) + 6I-(aq) + 8H+(aq) → 2MnO2(s) + 3I2(s) + 4H2O(l)

What is the equilibrium constant expression for the following reaction?
2Al(s) + 3Mn2+(aq) → 2Al3+(aq) + 3Mn(s)

Which of the following changes to the following reaction at equilibrium would result in a shift to the right?
COCl2(g) + HCl(g) CHCl3(l) + ½O2(g)
a decrease in pressure
removal of COCl2
none of these
an increase in the volume of the reaction vessel
Generally the addition of a product or the removal of a reactant to a system at equilibrium will result in a shift to the left, whereas the addition of a reactant or the removal of a product to a system at equilibrium will result in a shift to the right. The removal of COCl2 (a reactant) will therefore result in a shift to the left and can be eliminated.
An increase in pressure (or a reduction in volume) will result in a shift to the side that has the fewer number of moles of gas, whereas a decrease in pressure (or an increase in volume) will have the opposite affect. So both an increase in the volume of the reaction vessel and a decrease in pressure will have the same result, being a shift to the side with more moles of gas which is the reactants in this case (a shift left). These two choices are thereby eliminated and therefore “none of these” is the correct answer.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!

This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; the carboanion is sp2 hybridized as it is part of the conjugated system being one atom away from pi electrons)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (lone pair counts) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; the sulfur atom is sp2 hybridized as it is part of the conjugated system being one atom away from pi electrons)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (one lone pair counts) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; the oxygen atom is sp2 hybridized as it is part of the conjugated system being one atom away from pi electrons)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (one lone pair counts) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; the nitrogen atom is sp2 hybridized as it is part of the conjugated system being one atom away from pi electrons)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (lone pair counts) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; both nitrogen atoms are sp2 hybridized)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (one lone pair counts) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!
As there is an sp3 hybridized atom in the ring, this compound doesn’t satisfy the 2nd criterion of aromatic compounds and is therefore non-aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!
As this compound isn’t planar it doesn’t satisfy the 3rdcriterion of aromatic compounds and is therefore non-aromatic.
Which of the following is aromatic?

I only Correct!
I is aromatic as it is cyclic and conjugated, has no sp3 hybridized atoms, is planar, and has 6 π electrons (a 4N+2 #). The sulfur may appear sp3 hybridized upon initial inspection but due to resonance is really sp2 hybridized with 1 of its lone pairs in a ‘p’ orbital therefore counting as 2 additional π electrons for a total of 6.
Which of the following is aromatic?

I, II, and III Correct!

Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is
1) cyclic
2) completely conjugated (no sp3 atoms in the ring; the carbocation is sp2 hybridized)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar
4) has 2 pi electrons which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
This compound is:
1) cyclic
2) completely conjugated (no sp3 atoms in the ring)
3) planar (all rings 7 atoms or less can be planar; more than 7 atoms may or may not be planar)
4) has 6 pi electrons (lone pair doesn’t count as the nitrogen already has pi electrons i.e. pi bond) which is an aromatic number (4N+2)
This compound satisfies all the criteria and is aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!
As this compound isn’t cyclic it doesn’t satisfy the 1stcriterion of aromatic compounds and is therefore non-aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!

As there is an sp3 hybridized atom in the ring, this compound doesn’t satisfy the 2nd criterion of aromatic compounds and is therefore non-aromatic.
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!
Is the following compound aromatic, anti-aromatic, or non-aromatic?

aromatic Correct!
Is the following compound aromatic, anti-aromatic, or non-aromatic?

non-aromatic Correct!
As there are sp3 hybridized atoms in the ring, this compound doesn’t satisfy the 2nd criterion of aromatic compounds and is therefore non-aromatic.
Which of the following is a major product of the following reaction?

This EAS reaction is called Friedel-Crafts acylation. In an EAS reaction, we typically only add the electrophile once unless multiple equivalents are explicitly added and therefore choices B and D are eliminated. The OCH3 is electron donating and activating (and an ortho/para director) whereas the CHO is electron withdrawing and deactivating. Therefore the benzene on the right is more reactive and the acetyl group will be directed to one of the ortho positions (the para already has a substituent) and so choice A is the correct answer.

What is the major product of the following reaction?

The reagents listed add a sulfonate group to a benzene ring in an EAS reaction. The cyano group is a meta director (don’t forget that it’s the group already on the benzene in the reactant that does the directing) and therefore the major product is

What is/are the product(s) of the following reaction?


What is the major product of the following reaction?

The reagents listed add a nitro group (-NO2) to a benzene ring in an EAS reaction. The methoxy group is an ortho/para director (don’t forget that it’s the group already on the benzene in the reactant that does the directing) and therefore the two major products are


is an EAS reaction resulting in bromination. The carboxyl group (-COOH) is electron withdrawing and a meta director and therefore B is the correct answer.

What is the major product of the following reaction?


What is the major product of the following reaction?


What is the major product of the following reaction?



The reagents listed are one way of preparing chromic acid, an oxidizing agent for primary and secondary alcohols. Chromic acid converts primary alcohols to carboxylic acids and secondary alcohols to ketones. Our reactant here is a secondary alcohol and is therefore oxidized to form the following ketone:

Chromic Acid
Chromic acid oxidizes primary alcohols and aldehydes to
carboxylic acids and oxidizes secondary alcohols to ketones. It is tertiary alcohols that do not undergo
normal oxidation by chromic acid.

The reaction of an alcohol with H2SO4
is an elimination reaction that yields the Zaitsev product (most substituted
alkene possible) which is:


The tosylate group is a good leaving group and the 2nd step is an SN2 reaction (indicated by the presence of a strong leaving group and the addition of a strong nucleophile in a polar aprotic solvent) which substitutes a cyano group for the tosylate group with inversion of configuration:


The reaction of an alcohol with PBr3 is a substitution where the net result is the substitution of a Br atom for the OH group. It proceeds through the SN2 mechanism so if the OH group is on a chiral center (as in this case) inversion takes place leading to the following as the correct answer:


This reaction is an example of the cleavage of an ether with HBr. Reactions with 1 equivalent will convert the ether into an alkyl halide and an alcohol. But with excess HBr the alcohol is typically converted into an alkyl halide as well. The following diagram shows the ultimate fate of each portion of the molecule. Each of the C-O bonds is cleaved. The oxygen ends up as water and each of the carbon chains as alkyl bromides.




This reaction is an example of the cleavage of an ether with HBr. Reactions with 1 equivalent will convert an ether into an alkyl halide and an alcohol. But with excess HBr the alcohol is typically converted into an alkyl halide as well; however phenyl ethers are an exception as even if we protonate the hydroxyl group to from water (a good leaving group) it still won’t be able to leave an sp2 hybridized carbon to do the substitution reaction. The following diagram shows the fate of the different portions of the molecule.






Thionyl chloride (SOCl2) converts a carboxylic acid into an acid chloride which undergoes nucleophilic acyl substitution with an alcohol to form an ester.









How many unique products are there in the following reaction?

The molecule above is a triglyceride and will undergo basic hydrolysis of the 3 esters (saponification) breaking the bonds indicated below and forming 3 carboxylate salts and glycerol. But 2 of the fatty acid chains are identical and thus 2 of the carboxylate salts will be identical and therefore only 3 unique products are formed.




As the cyano (CN) groups are cis in the reactant, they will remain cis in the product (choice B).
*Note - trans dienes are able to spontaneously convert to cis conformation by rotation about the central single bond, provided that the ‘R’ groups either side of the central single bond are not too bulky (i.e. phenyl or t-butyl groups would block such a conversion). In this case we have only hydrogens either side so the steric interaction in cis conformation is low, and the trans diene would have to convert to cis before Diels-Alder can occur. Though the trans diene conformation is more stable than cis, the Diels-Alder reaction forms 2 sigma bonds from 2 pi bonds, which is favorable, so there is still a sufficient driving force for the reaction to occur.



