Organ Systems Exam 3 Flashcards
- Describe the structure and function of the serous pericardium. What is a bursa? Describe the fibrous pericardium and what it attaches to.
- Describe the structure and function of the serous pericardium. What is a bursa? Describe the fibrous pericardium and what it attaches to.
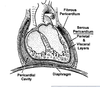
Pericardium – two types of CT sheaths around the heart
Fibrous pericardium:
• Thick CT surrounding heart
• Adhered to sternum and diaphragm
Serous pericardium: pericardial cavity lubricant reduces friction.
Fist-in-a-balloon model
• Parietal layer: outer layer adhered to inner surface of fibrous pericardium
• Visceral layer: invests heart (also called epicardium)
Bursa - a closed, fluid filled sack that acts as a buffer between 2 structures (protective).
Muscles of heart
Muscles of heart
In ventricles
Bulbospiral muscle
A spiral muscle that wraps around ventricles
“wrings out” blood
Trabeculae carnae
Thick ventricular cardiac muscle fibers
Location
On inner surface of ventricle
I.e., Run vertically down ventricle
Function
Aid in contraction upwards (?)
In atria
Pectinate muscles
Thin muscle fibers
Found in atria
Valves
Valves
Function
Maintain one-way blood flow through heart
Two main types
Atrioventricular valves
Tricuspid
On right side
Bicuspid
On left side
Semilunar valves
Pulmonary semilunar valve
Aortic semilunar valve
In right side of heart
Tricuspid valve
Right AV valve
Pulmonary semilunar valve
In left side of heart
Bicuspid valve
Left AV valve
AKA mitral AV valve
Aortic valve
AKA aortic semilunar valve
Between atria and ventricles
Atrioventricular valves
Tricuspid
Bicuspid
Between ventricles and blood vessels
Semilunar valves
Pulmonary semilunar valve
Aortic semilunar valve
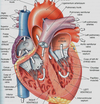
Picture of Heart
Right Ventricle Structure & Function
Right Ventricle structure and function
Tricuspid valve
General description
A three-cusped valve supported by chordate tendinae attached to papillary muscles in right ventricle of heart
Location
Between right atrium and right ventricle
Cusp number
3 cusps
I.e., tricuspid
Structural supports
Papillary muscles
Extension of trabeculae carnae
I.e., thick superficial ventricle muscle fibers
Attach from trabeculae carnae to cusps via chordate tendinae
Chordae tendinae
Bundles of connective tissue
Connect valves to papillary muscles
Ensures valve cusps do not prolapse!
I.e., that valve remains one-way into ventricle only
Moderator band
Part of bundle of His
Attaches inferiorly to papillary muscles
Source of blood:
Right atria
Anatomical features
Trabeculae carnae
I.e., Dense smooth muscle bundles in wall
Give ventricle a textured surface
Exit of blood:
Via pulmonary semilunar valve
To lungs
Via pulmonary trunk
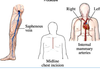
- Where are blood vessels for bypass surgeries taken from?
- Where are blood vessels for bypass surgeries taken from?
Treatments for blocked coronary arteries
•
Coronary bypass: shunt from the aorta to the affected coronary artery beyond the point of occlusion (atherosclerosis; angina). Bypasses are taken from blood vessels in other parts of the body
- Saphenous leg veins for smaller arteries, eg marginal
- Internal thoracic arteries for larger arteries, eg. anterior descending.
- What are the main features of angina pectoris in terms of coronary arteries? What is collateral circulation of the heart? Is it helpful during an acute heart attack?
- What are the main features of angina pectoris in terms of coronary arteries? What is collateral circulation of the heart? Is it helpful during an acute heart attack?
Angina pectoris occurs when cardiac workload and resultant myocardial O2 demand exceed the ability of coronary arteries to supply an adequate amount of oxygenated blood, as can occur when the arteries are narrowed. Narrowing usually results from atherosclerosis but may result from coronary artery spasm or, rarely, coronary artery embolism. Acute coronary thrombosis can cause angina if obstruction is partial or transient, but it usually causes MI.
ANGINA PECTORIS
- Pain, pressure, heaviness, tightness, squeezing, burning, or choking associated with lack of oxygen precipitated by exertion or emotional stress.
- Painful vasospasms can also occur in response to smoking: Prinzmetal angina
- Angina etymology: Latin angina (“infection of the throat”) from the Greek ἀγχόνη ankhonē (“strangling”), and the Latin pectus (“chest”)…translated as “a strangling feeling in the chest”. (from wikipedia)
- Treatment is to either vasodilate arteries (nitroglycerin: converted to NO via aldehyde dehydrogenase) or reduce load of heart (beta blocker: reduce cardiac output)
Collateral circulation in the heart
- Gradual ischemia (as in angina) induce formation of collateral arteries that compensate for coronary artery occlusion
- Collateral formation is gradual over months to years in the case of advancing disease such as angina, but can be overwhelmed by rapid onset of major obstruction and myocardial infarction
Cardiac collateral circulation[edit]
Another example (in humans) is where a person suffers an acute myocardial infarction (heart attack). Collateral circulation in the heart tissue will sometimes bypass the blockage in the main artery and supply enough oxygenated blood to enable the cardiac tissue to survive and recover.
- Describe the origin and distribution of the major coronary arteries and veins. Blood perfusion of the myocardium occurs during diastole – Why?
- Describe the origin and distribution of the major coronary arteries and veins. Blood perfusion of the myocardium occurs during diastole – Why?
Coronary arteries Right & Left Coronary arteries stem from aortic sinuses and give off descending branches.
Left coronary artery branches
- Anterior descending (interventricular)
- Circumflex (anastomoses with right coronary artery)
Right coronary artery branches • Marginal (inferior margin of heart) • Posterior descending (interventricular)
>>Blood perfusion of the myocardium occurs during diastole when the heart relaxes since at this time the semilunar valves close, which increases the pressure of the valves.
- What produces the heart sound: how do they relate to valves and the stages of systole and diastole?
- What produces the heart sound: how do they relate to valves and the stages of systole and diastole?
The first sound of the heart occurs during systole (“squeezing of heart) when the tricuspid & bicuspid (mitral) valves close and the semilunar valves open. The second sound occurs when the aortic & pulmonary semilunar valves close & the tricuspid and bicuspid valves open during dystole (the “relaxation of the heart”):
- Describe the anatomy and function of semilunar valves. Where are they located?
- Describe the anatomy and function of semilunar valves. Where are they located?
The semilunar valves include the aortic semilunar valve (between the left ventricle and the aorta) & the pulmonary semilunar valve (between the right ventricle and the pulmonary artery). They have three cusps (or “leaflets”). During ventricular systole, the pressure rises in the left ventricle and when it is greater than the pressure in the aorta, the aortic valve opens, allowing blood to exit the left ventricle into the aorta. When ventricular systole ends, a decrease in pressure causes the valve to close.
Meanwhile, the pulmonary semilunar valve also opens during ventricular systole (and also has three cusps). It opens ins ventricular systole due to an increase in pressure in the right ventricle. When the pressure decreases at the end of ventricular systole, the pressure in the pulmonary artery will close the pulmonary valve.
- Which chamber has the thickest myocardium? Any idea why this is the case?
- Which chamber has the thickest myocardium? Any idea why this is the case?
The left ventricle has the thickest myocardium since it needs to pump blood to the rest of the body. Meanwhile, the right ventricle only needs to pump blood to the lungs, so there isn’t as much of a need to have a thick myocardium.
- What are pectinate muscles and auricles? What role do they play in circulation through the heart?
- What are pectinate muscles and auricles? What role do they play in circulation through the heart?
The Pectinate (atrial) muscles and the vestigial auricle contract to push blood into ventricle
- Describe the anatomy and function of the tricuspid and bicuspid valves. Where are they located? How do they relate to systole and diastole? What are papillary muscles and how do they relate to trabeculae carneae?
- Describe the anatomy and function of the tricuspid and bicuspid valves. Where are they located? How do they relate to systole and diastole? What are papillary muscles and how do they relate to trabeculae carneae?
The tricuspid valve is found between the right atrium and the right ventricle. It has three cusps, and there are about three papillary muscles that pull the chordae tendineae that are connected to the valve cusps.
Meanwhile, the bicuspid (mitral) valve has two cusps) and is found between the left atrium and the left ventricle. During systole (ventricular contraction, or “squeezing of the heart”), the AV valves (including the tricuspid and bicuspid (or mitral) valves) close, and the semilunar valves (including the aortic semilunar valve & pulmonary semilunar valve) open. Meanwhile, during diastole (filling of the heart), the AV valves close and the semilunar valves open.
>>The first sound is the closing of the AV valves, while the second sound from the heart is the closing of the semilunar valves.
- Where and what is the fossa ovalis? Where is the opening of the coronary sinus? What is its significance in coronary circulation?
- Where and what is the fossa ovalis? Where is the opening of the coronary sinus? What is its significance in coronary circulation?
The fossa ovalis is found between the right and left atriums. It closes when a baby is born, and when a baby is in its mother’s womb, the fossa ovalis is open and it is the pathway for deoxygenated blood that is transported from the mother to the fetus.
The coronary sinus is also found in the right atrium, and it brings in deoxygenated blood from the heart to the right atrium.
- What blood enters the right and left ventricles and where is it pumped after that?
- What blood enters the right and left ventricles and where is it pumped after that?
Blood enters the right atrium through the inferior & superior vena cavas, and then it travels through the tricuspid valve to the right ventricle. After this, it goes through the Pulmonary semilunar valve to become oxygenated.
The entire right side of the heart transports deoxygenated venous blood to the lungs.
-2. Describe the anatomy of the heart conduction system. What is the significance of the AV node in conduction from atrium to ventricle? Are any parts of the conduction system neural?
-2. Describe the anatomy of the heart conduction system. What is the significance of the AV node in conduction from atrium to ventricle? Are any parts of the conduction system neural?
The AV node is located in the base of the right atrium, and it is the regulator of conduction into the ventricles. The AV node sends fibers through the CT ring surrounding the tricuspid valve. The Bundle of His is the only conducting pathway between the atria and the ventricles. The AV node has the slowest conducting velocity since it has fewer gap junctions, which creates delays between the atrial and ventricular contraction, and gives time for filling.
- How does cardiac muscle differ from skeletal and smooth muscle in terms of cell structure and connections between cells?
- How does cardiac muscle differ from skeletal and smooth muscle in terms of cell structure and connections between cells?
Cardiac muscles (myocardium) are small cells with single nucleus, some branched. They also have sarcomeres similar to skeletal muscle: striated myocardium. They have T tubules, and a sarcoplasmic reticulum. The primary structure of cardiac muscle is myosin & actin, however unlike skeletal muscle, cardiac muscle are brach-like instead of linear. T tubules are bigger and wider and track laterally to the Z disks. Cardiomyocytes are also connected to each other by intercalated disks that allow for rapid transmission of electrical impulses. Desmosomes interconnect cardiac cells, transmit contraction forces and hold the tissue together. Meanwhile, gap junctions, are especially important in cardiac cells for communication, and allow for the passage of ions and depolarizing current.
- Describe each phase of the cardiac cycle in terms of: ECG waves, ventricular, atrial and aortic pressures (where appropriate), isometric versus isotonic contraction, valve actions, heart sounds.
- Describe each phase of the cardiac cycle in terms of: ECG waves, ventricular, atrial and aortic pressures (where appropriate), isometric versus isotonic contraction, valve actions, heart sounds.
Late Ventricular Diastole (tan): BEFORE T = 0
Both sets of chambers relaxed, passive ventricular filling.
Atria are in systole.
Atrial Systole (pink): T = 0 msec to T = 100 msec
Atrial contraction forces a small amount of additional blood into ventricles.
ECG:
The beginning of atrial systole corresponds with the P wave (T = 0).
The peak of the QRS complex corresponds to the end of atrial systole, and the beginning of ventricular systole (T = 100).
Ventricles are still in diastole:
End-diastolic volume (EDV) - The maximum amount of blood in the ventricles occurs at the end of ventricular relaxation (~135 mL).
Ventricular Systole (purple): T = 100 msec to T = 350 msec
Phase I: Isovolumic ventricular contraction - First phase of ventricular contraction pushes AV valves closed but does not create enough pressure to open semilunar valves. (isovolumic = isometric contraction)
Mitral valve closes at beginning of this phase, as left ventricular pressure begins to surpass left atrial pressure.
The first heart sound occurs during this phase.
ECG - The ST phase occurs during this phase.
Pressures -
Phase II: Ventricular ejection - as ventricular pressure rises and exceeds pressure in the arteries, the semilunar valves open and blood is ejected.
Aortic valve opens at beginning of this phase, as left ventricular pressure reaches aortic pressure.
End-systolic volume (ESV) - The minimum amount of blood in the ventricles occurs after ventricular ejection (~65 mL).
ECG - The T wave occurs at the end of this phase.
Atria are in diastole.
Ventricular Diastole (tan): T = 350 msec to T = 800 msec
Phase I: Isovolumic ventricular relaxation - As ventricles relax, pressure in ventricles drops, blood flows back into cups of semilunar valves and snaps them closed. (isovolumic = isometric contraction)
Aortic valve closes at beginning of this phase, as left ventricular pressure dips below aortic pressure.
The second heart sound occurs during this phase.
Phase II: Late Ventricular Diastole - both sets of chambers relaxed, passive ventricular filling.
Mitral valve opens at beginning of this phase, as left ventricular pressure dips below left atrial pressure.
Atria remain in diastole for part of ventricular diastole.
Atrial Systole (pink): T = 800 msec to 900 msec
Beginning of new cycle.
Ventricles are still in diastole.
***Tip to help remember systole/diastole:
Systole = Squeezing blood out
Diastole = Dilation lets blood in
***Also, remember that during afterload, different pressures are needed to eject blood
- Isometric contraction is used to reach aortic pressure level - isometric = unchanging muscle length
- Isotonic contraction is used during ejection - isotonic = equal tension/weight with changing muscle length

- Describe the conduction events during the: Pwave, P-Q interval, R wave, S-T interval, T wave. When in the ECG is the ventricle fully repolarized?
- Describe the conduction events during the: Pwave, P-Q interval, R wave, S-T interval, T wave. When in the ECG is the ventricle fully repolarized?
As you can see in the diagram below, the ventricles are fully repolarized after the T wave, and repolarization creates the same polarity as the action potential.

- What impact does the slow conduction of the AV node have on the cardiac cycle?
- What impact does the slow conduction of the AV node have on the cardiac cycle?
Slow conduction of the AV node allows for a time gap between atrial and ventricular contraction, giving time for filling.
- Describe the conduction AP and activity of ion channels. How does this differ from contraction APs? What happens to the heart rate if there is a bundle block in the ventricle?
- Describe the conduction AP and activity of ion channels. How does this differ from contraction APs? What happens to the heart rate if there is a bundle block in the ventricle?
There are two different types of myocardial action potentials:
The conduction action potential (which is what is being sent down the SA node to the AV node, the bundle of his, etc.) is exemplified by the conduction APs of the SA and AV nodes, which are autorhthmic. No signaling factors are needed. A slow L type calcium channel produces rapid onset. K+ channels hyperpolarize the cell, and a funny Na+ channel, Naf, opens with hyperpoplarization and produces slow depolarization that spontaneously generates the next AP - autorhythmicity. Similar rhythms can be generated in Purkinje fibers and myocardial cells. If there is a bundle block to the ventricle, any site can take over the heart rate (which is what the SA node usually does). The SA node also has the fastest auto rhythm (ie. phase 4 auto rhythm).
The conduction AP differs from contraction Action potentials, which are made by the myocardial cells (including the atria, ventricles, and purkinje fibers). Contraction Action Potentials are extended plateaus, and are elicited by impulses from SA and AV nodes. Myocardial contraction is triggered by long duration action potential in a similar time frame.
- How do sympathetic and parasympathetic activity impact heart rate? What receptors and ion channels are affected in each case? What is the difference between the fast and slow effects of parasympathetic activity? What is vagal tone and what is its contribution to the resting heart rate? What is the relative contribution of the sympathetic activity to the resting heart rate?
I
- How do sympathetic and parasympathetic activity impact heart rate? What receptors and ion channels are affected in each case? What is the difference between the fast and slow effects of parasympathetic activity? What is vagal tone and what is its contribution to the resting heart rate? What is the relative contribution of the sympathetic activity to the resting heart rate?
Parasympathetic (“rest & digest”) activity - stimulates the vagus nverve, reducing the heart’s activity. It slows the heart rate, conduction at the AV node is delayed, the force of contraction decreases, and its excitability decreases. Preganglionic neurons from the brain stem project, and postganglionic neurons in the myocardium stimulate muscarinic recetors in nodes. Parasympathetic activity affects the nodal cells. ACh stimulates M2 muscarinic receptors on: 1. SA node to decelerate the heart (right vagus). 2. AV node to reduce AV conduction (left vagus).
Fast Action parasympathetic activity: M2 receptors hyperpolarize the membrane by opening K+ channels. It slows depolarization by inhibiting T type Ca++ and funny Na+ channels. >>Fast action exerts quick beat by beat control of SA and AV function.
Slow Action parasympathetic effects on nodal cells: >M2 receptors activate inhibitory G proteins (Gi) that reduce cAMP synthesis. Less cAMP inhibits funny Na + channel; this reduces the rate of membrane depolarization. The slower process sets the “vagal tone” of the HR and competes with sympathetic acceleration of the heart.
Vagal tone: Parasmpathetic neurons DOMINATE regulation of heart rate. Vagal tone and cAMP levels. Adrenergic (sympathetic) vs. muscarinic (parasympathetic) pathways. The SA node “intrinsic rate” of about 100/min (as in heart transplant). Vagal activity reduces this to about 70 for resting heart rate. The resting sympathetic activity is low and when stimulated generates a small (but still functionally significant) increase in HR compared to parasympathetic.
Sympathetic Activity on Heart Rate: >>Effects on nodal cells (slow action) –>NE (Sympathetic) and Epi (adrenal medulla) stimulate B1 receptors on:
>SA node to increase heart rate
>AV node to facilitate AV conduction
B1 receptors increases the activity of both Ca++ and funny Na+ channels via cAMP and PKA. Stimulation of funny Na+ is what increases heart rate by accelerating the depolarization toward threshold (since it comes into the cells). Sympathomimetics (eg ephedra) act on B1 receptors.
Parasympathetic effect on nodal cells (picture)

- How do sympathetic and parasympathetic activity impact heart rate? What receptors and ion channels are affected in each case? What is the difference between the fast and slow effects of parasympathetic activity? What is vagal tone and what is its contribution to the resting heart rate? What is the relative contribution of the sympathetic activity to the resting heart rate?
II
- How do sympathetic and parasympathetic activity impact heart rate? What receptors and ion channels are affected in each case? What is the difference between the fast and slow effects of parasympathetic activity? What is vagal tone and what is its contribution to the resting heart rate? What is the relative contribution of the sympathetic activity to the resting heart rate?
Parasympathetic (“rest & digest”) activity - stimulates the vagus nverve, reducing the heart’s activity. It slows the heart rate, conduction at the AV node is delayed, the force of contraction decreases, and its excitability decreases. Preganglionic neurons from the brain stem project, and postganglionic neurons in the myocardium stimulate muscarinic recetors in nodes. Parasympathetic activity affects the nodal cells. ACh stimulates M2 muscarinic receptors on: 1. SA node to decelerate the heart (right vagus). 2. AV node to reduce AV conduction (left vagus).
Fast Action parasympathetic activity: M2 receptors hyperpolarize the membrane by opening K+ channels. It slows depolarization by inhibiting T type Ca++ and funny Na+ channels. >>Fast action exerts quick beat by beat control of SA and AV function.
Slow Action parasympathetic effects on nodal cells: >M2 receptors activate inhibitory G proteins (Gi) that reduce cAMP synthesis. Less cAMP inhibits funny Na + channel; this reduces the rate of membrane depolarization. The slower process sets the “vagal tone” of the HR and competes with sympathetic acceleration of the heart.
Vagal tone: Parasmpathetic neurons DOMINATE regulation of heart rate. Vagal tone and cAMP levels. Adrenergic (sympathetic) vs. muscarinic (parasympathetic) pathways. The SA node “intrinsic rate” of about 100/min (as in heart transplant). Vagal activity reduces this to about 70 for resting heart rate. The resting sympathetic activity is low and when stimulated generates a small (but still functionally significant) increase in HR compared to parasympathetic.
Sympathetic Activity on Heart Rate: >>Effects on nodal cells (slow action) –>NE (Sympathetic) and Epi (adrenal medulla) stimulate B1 receptors on:
>SA node to increase heart rate
>AV node to facilitate AV conduction
B1 receptors increases the activity of both Ca++ and funny Na+ channels via cAMP and PKA. Stimulation of funny Na+ is what increases heart rate by accelerating the depolarization toward threshold (since it comes into the cells). Sympathomimetics (eg ephedra) act on B1 receptors.
Picture: Parasympathetic effects on nodal cells, slow action.

- Describe the key features of the ventricular contraction AP and how they relate to the different ion channel activities. How do calcium blockers function to reduce blood pressure in eg. hypertension?
- Describe the key features of the ventricular contraction AP and how they relate to the different ion channel activities. How do calcium blockers function to reduce blood pressure in eg. hypertension?
In contraction action potential, the Na+ current (voltage gated) starts cardiac Action Potential, but inactivates. The slow L type Ca channels produce long duration Action potential with plateau: > Assisted by decrease in K+ outward current, >Inward Ca current triggers Ca++ release from Sarcoplasmic Reticulum.
Also, Calcium blockers (nifedipine, verapamil, etc.) block L type channels to reduce cardiac contractility (through decreasing calcium release in myocardium). This can be used to treat hypertension!

- Describe how the contractile AP causes the release of Ca from the SR. How does this mechanism differ from that of skeletal and smooth muscle? How does the cardiac muscle cell relax after contraction?
- Describe how the contractile AP causes the release of Ca from the SR. How does this mechanism differ from that of skeletal and smooth muscle? How does the cardiac muscle cell relax after contraction?
An AP opens the L-type Ca channels in the T tubule adjacent to one SR (diad). The inward calcium flux binds with RyR “feet” and opens SR Ca channel in the terminal cisternae. The trigger calcium acts solely to open (“trigger”) the SR release of CA. In BOTH skeletal and cardiac muscle, it is the incoming AP that initiates the release of SR Ca++. Calcium from SR initiates cross bridge formation, and calcium is reabsorbed into SR via SERCA and phospholambin. Also, the AP reaches Ca++ channels in T tubule via gap junctions from neighboring cells & autorhythmic mechanisms.
After contraction: The cardiac cell relaxes through the cessation of AP or autonomic stimulation. This causes calcium uptake into SR via Ca-ATPase. Calcium effluxes from the cell via Na+/Ca++ exchanger, which allows for 3 Na in, 1 Ca out. Na goes down its concentration gradient created by the Na pump.














