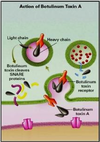
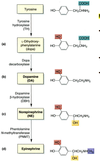
Organ Systems Exam 2! Flashcards
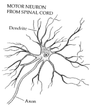
- What is the difference between axons and dendrites?
- What is the difference between axons and dendrites?
Various examples of
neurons
• one axon, but many
dendrites
Neurons
Basic structural features
• Cell body
• Axon
• Dendrites
• Synapse
Nomenclature
PNS CNS
Cell bodies Ganglion Nucleus
Axons Nerve Tract
• Nucleus also sometimes called a body
• Tract also called fasciculus, funiculus,
peduncle, column, lemniscus,
commissure or capsule
- What are Schwann cells and how do they interact with neuronal axons?
- What are Schwann cells and how do they interact with neuronal axons?
Glia
All neurons in the PNS are
surrounded by Glia (Schwann cells)
• Schwann cells:
• PNS glial cell surrounds all peripheral
nerve axons and cell bodies
• Gaps between cells are the nodes of
Ranvier
• Myelinated vs unmyelinated axons (more
of this later)

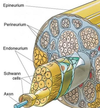
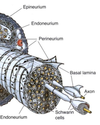
- What are the three CT layers surrounding a large nerve? What is the basal lamina in nerves? What is its significance in regeneration? 1st Question!
3. What are the three CT layers surrounding a large nerve? What is the basal lamina in nerves? What is its significance in regeneration?
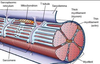
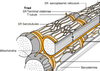
Connective tissue around nerves
Epineurium
Perineurium
• Fascicles
Endoneurium
- What are the three CT layers surrounding a large nerve? What is the basal lamina in nerves? What is its significance in regeneration? 2nd part!
- What are the three CT layers surrounding a large nerve? What is the basal lamina in nerves? What is its significance in regeneration?
Fascicle
• UMF (unmyelinated) and MF
(myelinated) fibers; V vessel; F
fibroblast
Endoneurium within fascicle
• Matrix around glial cells form
endoneurial tubes
Basal Lamina
• secreted by Schwann cells; interface
between Schwann cells and
endoneurium
• contains laminin, promotes growth
and regeneration of axons and glia
- What are nervi nervorum and what do they innervate? What are vasa nervorum and what do they supply? Distinguish between nerve trunk pain and dysesthetic pain.
- What are nervi nervorum and what do they innervate? What are vasa nervorum and what do they supply? Distinguish between nerve trunk pain and dysesthetic pain.
[*] Nervi nervorum: local nerves to CT of nerves
[*] Vasa nervorum: local blood vessels to nerves
Nervi Nervorum
• nerve trunk pain
• dysesthetic pain
>>Nerves to the nerves.
Vasa nervorum
• First degree neuropathic injury due to
temporary compression of
nerves/vessels causes numbness
>>EX: Your arm goes to sleep after leaning against the nerve while sleeping.
Nerve trunk pain: local pain
Dysesthetic pain - when you hit your funny bone and it shoots out to your finger. You’ve created an artificial sense of pain from your finger. It’s called line labeling. If you stimulate a neuron along this length, you’ll notice the same sensation.
- Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root? I
- Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root?
- DORSAL ROOT GANGLION
- cluster of cell bodies of sensory neurons
- pseudo-unipolar neuron
- central and peripheral branches
- SYMPATHETIC GANGLIA TRUNK/CHAIN
- perpendicular to peripheral nerves
- postganglionic neuron cell bodies (see below)
- grey and white rami5. Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root?
Internal structure of spinal cord
Gray matter
• Ventral (Anterior) horn
• Dorsal (Posterior) horn
White matter
• Axons
• Myelin “whitens” the tissue.
PERIPHERAL NERVES
• ROOTLETS converge into the ROOTS
• DORSAL and VENTRAL ROOTS unite to form SPINAL NERVE
• SPINAL NERVE splits into RAMI
• Dorsal ramus innervates deep back muscles and overlying skin
• Ventral ramus innervates remaining muscles, skin, etc.
• PERIPHERAL NERVE = dorsal and ventral rami;
• PNS: nerves outside the brain and spinal cord

- Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root? II
- Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root?
- DORSAL ROOT GANGLION
- cluster of cell bodies of sensory neurons
- pseudo-unipolar neuron
- central and peripheral branches
- SYMPATHETIC GANGLIA TRUNK/CHAIN
- perpendicular to peripheral nerves
- postganglionic neuron cell bodies (see below)
- grey and white rami5. Distinguish the following: dorsal/ventral horn, dorsal/ventral root, dorsal/ventral rami. What structures are located in the dorsal root? What are in the ventral root?
Internal structure of spinal cord
Gray matter
• Ventral (Anterior) horn
• Dorsal (Posterior) horn
White matter
• Axons
• Myelin “whitens” the tissue.
PERIPHERAL NERVES
• ROOTLETS converge into the ROOTS
• DORSAL and VENTRAL ROOTS unite to form SPINAL NERVE
• SPINAL NERVE splits into RAMI
• Dorsal ramus innervates deep back muscles and overlying skin
• Ventral ramus innervates remaining muscles, skin, etc.
• PERIPHERAL NERVE = dorsal and ventral rami;
• PNS: nerves outside the brain and spinal cord

- What is the structure of sensory neurons and how do they differ from other neurons?
- What is the structure of sensory neurons and how do they differ from other neurons?
- DORSAL ROOT GANGLION
- cluster of cell bodies of sensory neurons
- pseudo-unipolar neuron
- central and peripheral branches
- SYMPATHETIC GANGLIA TRUNK/CHAIN
- perpendicular to peripheral nerves
- postganglionic neuron cell bodies (see below)
- grey and white rami
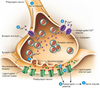
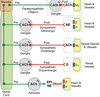
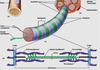
Constituents of a Peripheral Nerve
Sensory neurons (blue)
• convey info from periphery to CNS
• enters dorsal root & horn
• cell body in dorsal root ganglion
Motor neurons (red)
• convey info from CNS to skeletal muscle
• Cell body in ventral horn
• Axons exit ventral root
Sympathetic neurons (green)
• found in essentially all nerves
• preganglionic cell body in
intermediolateral column; projects axon
to sympathetic ganglion
• postganglionic cell bodies form the
sympathetic ganglia; project to smooth
muscle/glands, etc
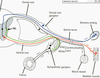
- Distinguish between the dorsal root ganglia and sympathetic ganglia.
- Distinguish between the dorsal root ganglia and sympathetic ganglia.
- DORSAL ROOT GANGLION
- cluster of cell bodies of sensory neurons
- pseudo-unipolar neuron
- central and peripheral branches
- SYMPATHETIC GANGLIA TRUNK/CHAIN
- perpendicular to peripheral nerves
- postganglionic neuron cell bodies (see below)
- grey and white rami
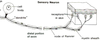
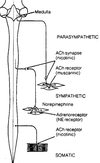
- What are the contents of a typical peripheral nerve? Trace the course of the preganglionic and postganglionic sympathetic neurons.
- What are the contents of a typical peripheral nerve? Trace the course of the preganglionic and postganglionic sympathetic neurons.
Constituents of a Peripheral Nerve
Sensory neurons (blue)
• convey info from periphery to CNS
• enters dorsal root & horn
• cell body in dorsal root ganglion
Motor neurons (red)
• convey info from CNS to skeletal muscle
• Cell body in ventral horn
• Axons exit ventral root
Sympathetic neurons (green)
• found in essentially all nerves
• preganglionic cell body in
intermediolateral column; projects axon
to sympathetic ganglion
• postganglionic cell bodies form the
sympathetic ganglia; project to smooth
muscle/glands, etc

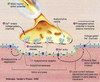
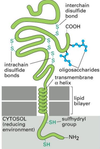
- How do phospholipids organize in the cell membrane? What does the presence of cholesterol have on the cell membrane? What are lipid rafts?
\9. How do phospholipids organize in the cell membrane? What does the presence of cholesterol have on the cell membrane? What are lipid rafts?
Electrical properties of neurons depend
on structural features of the cell membrane
Lipid bilayer
• Phospholipids (4 types) with polar heads and
two hydrophobic hydrocarbon tails
• Spontaneously organize into bilayered sphere
Cholesterol
• Major constituent of
membranes
• Determine permeability to
small water soluble
molecules.
Lipid rafts
• cholesterol & sphingolipids
• parcellate proteins
Permeability of cell membrane
• low permeability to H2O
• high permeability to O2 &
CO2

- How do alpha helical portions of transmembrane proteins relate to the formation of ion channels?
- How do alpha helical portions of transmembrane proteins relate to the formation of ion channels?
Transmembrane proteins
• Alpha helix: part of protein
within lipid membrane
• Multiple crossing: to form
aqueous pores
• Single crossing: to form
receptors for signals
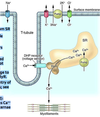
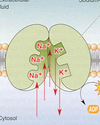
- Describe the equilibrium potential for ions like K and Na. Include: the role of ion flux and concentration gradients & the significance of concentration and electrical gradients. What does it mean to be in equilibrium in this case? What is the sodium-potassium pump and how does it work?
- Describe the equilibrium potential for ions like K and Na. Include: the role of ion flux and concentration gradients & the significance of concentration and electrical gradients. What does it mean to be in equilibrium in this case? What is the sodium-potassium pump and how does it work?
MEMBRANE POTENTIAL
• electrical potential across a cell membrane.
• Membrane potential is produced by
• ion flux
• concentration gradients
ION FLUX
• passage of ions through ion specific, cylindrical, membrane
protein-based ion channels.
• channels lined with negative charges permit passage of
positive, but not negative, ions.
CONCENTRATION GRADIENTS
• produced by Na-K, ATP-ase, the “Na-K Pump”.
• Na-K pump pumps 3 Na ions out &2 K ions in
against their concentration gradients
EQUILIBRIUM POTENTIAL
• membrane polarization created by a single ion.
• Outward flux of K+ down its concentration gradient produces a
negative charge on membrane interior
• The accumulation of negative charge on the interior
electrostatically pulls the K+ inward via an electrical gradient.
• Equilibrium state is reached when outward and inward K+ fluxes
are equal.
• This represents a STEADY STATE type of equilibrium.
Picture: Sodium Potassium pump
- Describe the membrane potential. Include: role of various ions, differences in permeability and concentration gradients, how it differs from the equilibrium potential. What is the dominant ion that determines the membrane potential at rest and why is it so? Why do K+ ions leak out of the cell?
- Describe the membrane potential. Include: role of various ions, differences in permeability and concentration gradients, how it differs from the equilibrium potential. What is the dominant ion that determines the membrane potential at rest and why is it so? Why do K+ ions leak out of the cell?
MEMBRANE POTENTIAL
• electrical potential across a cell membrane.
• Membrane potential is produced by
• ion flux
• concentration gradients
ION FLUX
• passage of ions through ion specific, cylindrical, membrane
protein-based ion channels.
• channels lined with negative charges permit passage of
positive, but not negative, ions.
CONCENTRATION GRADIENTS
• produced by Na-K, ATP-ase, the “Na-K Pump”.
• Na-K pump pumps 3 Na ions out &2 K ions in
against their concentration gradients
EQUILIBRIUM POTENTIAL
• membrane polarization created by a single ion.
• Outward flux of K+ down its concentration gradient produces a
negative charge on membrane interior
• The accumulation of negative charge on the interior
electrostatically pulls the K+ inward via an electrical gradient.
• Equilibrium state is reached when outward and inward K+ fluxes
are equal.
• This represents a STEADY STATE type of equilibrium.
MEMBRANE POTENTIAL, Vm
• Generated by the net flux of all ions.
• Typical membrane polarization from -90 to -55 mV.
• Na-K pump: adds -10mV because of net
outward Na+ pumping: ignore it here.
• Meaning of depolarization and hyperpolarization
GOLDMANN EQUATION quantifies Vm by weighting
each ionic flux with its permeability.
• Balance between motivation (concentration
gradient) and permission (channel permeability)
Ion leakage
• Net outward K+ and inward Na+ drift. Why?
• Recovered by pump
>>>K+ leaks out because there’s not enough potential E to pull it back in! Permeability of Na+ channels are very low. Membrane potential puts a diff. spin on what electrical dynamic of cell is. Sodium pump puts it back into cell happily!
Membrane potential, Vm
• Net driving force = concentration + electrical gradients
• Net flux=net driving force X permeability
K+ net flux is high
Na+ net flux is low (resting conditions)
Picture: Membrane potential
- What are the different types of ion channels and how are they activated? What are non-gated channels? Give an example.
- What are the different types of ion channels and how are they activated? What are non-gated channels? Give an example.
Ion permeability is
determined by gating
properties of channels
TYPE Voltage gated Ligand / Transmitter Mechanically gated
LOCATION Axon hillocks Neural or Skin, retina, etc
Nodes of Ranvier other cell surfaces
STIMULUS De- /Hyper- Neurotransmitters / Pressure, touch, light
Voltage gated ion channels
• Changes permeability in response to
depolarizing or hyperpolarizing charge Non-gated channels
• Normally no change in permeability in response to stimuli
• Up or down regulation can alter number or permeability of channels
• Non-gated K+ channels:
• ubiquitous
• high permeability; “K+ leak channels”
• primary determinant of the resting membrane potential.
- What are receptor potentials and how are they produced? How are they different from action potentials? Ist
- What are receptor potentials and how are they produced? How are they different from action potentials?
Receptor potential
• External stimuli open physically–
gated ion channels in axon terminals
• Receptor potential:
• Graded increase in Na+
permeability
• Ionic current opens voltage-gated
Na+ at the first node of Ranvier,
triggering all-or-none action
potentials
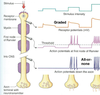
ACTION POTENTIALS
• Graded incoming positive
ionic current depolarizes
membrane to threshold level
of voltage.
• At threshold, rapid
depolarization to positive
value, near E (Na)
• Repolarization to initial
potential
• Hyperpolarization, near E (K)
- What are receptor potentials and how are they produced? How are they different from action potentials?
Receptor potential
• External stimuli open physically–
gated ion channels in axon terminals
• Receptor potential:
• Graded increase in Na+
permeability
• Ionic current opens voltage-gated
Na+ at the first node of Ranvier,
triggering all-or-none action
potentials
ACTION POTENTIALS
• Graded incoming positive
ionic current depolarizes
membrane to threshold level
of voltage.
• At threshold, rapid
depolarization to positive
value, near E (Na)
• Repolarization to initial
potential
• Hyperpolarization, near E (K)
- What are receptor potentials and how are they produced? How are they different from action potentials?
Receptor potential
• External stimuli open physically–
gated ion channels in axon terminals
• Receptor potential:
• Graded increase in Na+
permeability
• Ionic current opens voltage-gated
Na+ at the first node of Ranvier,
triggering all-or-none action
potentials
ACTION POTENTIALS
• Graded incoming positive
ionic current depolarizes
membrane to threshold level
of voltage.
• At threshold, rapid
depolarization to positive
value, near E (Na)
• Repolarization to initial
potential
• Hyperpolarization, near E (K)
Picture: Action potential
Shifts in polarization during the action potential are due to sequential
changes in voltage-gated Na and K channel permeability
A. Rest – both channels closed
B. Depolarization
• Na channel opens in response to depolarization (at threshold)
C. Repolarization
• Na channel spontaneously inactivates
• K channels opens
D. Hyperpolarization
• K channel still open; K flux now through both non-gated & volt-gated
channels
• Potential almost reaches E (K)

- Account for each phase of the action potential in terms of the Na and K channel activities. Why is the initial depolarization and overshoot of the action potential so large in magnitude compared to hyperpolarization?
- Account for each phase of the action potential in terms of the Na and K channel activities. Why is the initial depolarization and overshoot of the action potential so large in magnitude compared to hyperpolarization?
Shifts in polarization during the action potential are due to sequential
changes in voltage-gated Na and K channel permeability
A. Rest – both channels closed
B. Depolarization
• Na channel opens in response to depolarization (at threshold)
C. Repolarization
• Na channel spontaneously inactivates
• K channels opens
D. Hyperpolarization
• K channel still open; K flux now through both non-gated & volt-gated
channels
• Potential almost reaches E (K)

- Describe how raising or lowering the concentration gradient of K can change neuronal excitability. How can alterations in the Na channel affect neuronal excitability.
- Describe how raising or lowering the concentration gradient of K can change neuronal excitability. How can alterations in the Na channel affect neuronal excitability.
Channel population changes in
Na/K channel permeability
• Depolarization: activation of
most Na channels
• Repolarization
• Inactivation of Na
channels
• Slower opening of K
channels
• Hyperpolarization
• Opened K channels that
bring Vm closer to K
equilibrium potential
• Initial depolarization and overshoot of
an action potential is strong, because
Na influx has a high driving force
• Both concentration and electrical
gradients are in the same direction and
summate.
• This is why membrane potential almost
reaches the equilibrium potential for Na
at the peak of the action potential

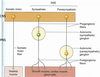
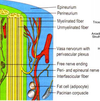
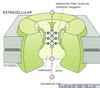
- Compare myelinated vs unmyelinated axons in terms of: Schwann cell configuration, action potential formation, action potential conduction. What is the difference between absolute and relative refractory periods? Describe the types of ion channels found in nodes of Ranvier and the intermodal regions of the axons.
- Compare myelinated vs unmyelinated axons in terms of: Schwann cell configuration, action potential formation, action potential conduction. What is the difference between absolute and relative refractory periods? Describe the types of ion channels found in nodes of Ranvier and the intermodal regions of the axons.
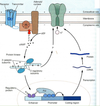
NEUROPATHY
Neural disease due to damage to either axons or glial cells, or both
Axonal degeneration
• symptoms progress from the hands and feet proximally
Demyelination
• degeneration of glia and the unraveling of myelin sheaths around axons
• Guillain-Barre: demyelination of mostly motor fibers by auto immune response to
infection, surgery, or immunization
• Contrasts with multiple sclerosis (MS), a demyelinating disease in the CNS
Negative symptoms
• loss of sensation, muscle weakness & atrophy
Positive symptoms
• Paresthesia
• Hyperalgesia
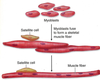
Unmyelinated axons Myelinated axons surrounded
surrounded by single by several layers of glial
layer of glial (Schwann) membrane which forms myelin.
Picture: myelinated axons
AP CONDUCTION IN
UNMYELINATED AXONS
• Continuous production of action
potentials along whole axon:
• Limits conduction velocity
• Maintains fidelity
• Voltage-gated Na+ and K+ ion
channels are distributed along the
whole axon
• Ionic current
REFRACTORY PERIOD in unmyelinated axons is
the period of time after an action potential during
which the membrane channels are less responsive
to stimuli
Absolute refractory period
• Na channels inactivated
Relative refractory period
• K channels open
This limits conduction to one direction and a
maximal AP frequency of 500/sec
AP CONDUCTION IN MYELINATED AXONS
• AP’s are only produced in nodes of Ranvier and
the axon hillock; only location of voltage-gated
channels
Axonal myelination increases AP
conduction velocity
• Electrotonic conduction through
internodal areas
• Amplitude of impulses decrease,
because of ionic current leak
• Sufficient depolarizing strength
initiates AP at the next node.
DEMYELINATION
• Common cause of neuropathy, esp. in autoimmune conditions like multiple sclerosis
and Guillain-Barre syndrome
• Glial cells unwind and decrease thickness of myelin, facilitating current leakage
through the membrane
• In demyelinated areas, AP conduction is slowed or halted because excess current
leaks out through non-gated K channels.
• This reduces the available current for the next node of Ranvier, eg.

- What impact does demyelination have on action potential conduction?
- What impact does demyelination have on action potential conduction?
NEUROPATHY
Neural disease due to damage to either axons or glial cells, or both
Axonal degeneration
• symptoms progress from the hands and feet proximally
Demyelination
• degeneration of glia and the unraveling of myelin sheaths around axons
• Guillain-Barre: demyelination of mostly motor fibers by auto immune response to
infection, surgery, or immunization
• Contrasts with multiple sclerosis (MS), a demyelinating disease in the CNS
Negative symptoms
• loss of sensation, muscle weakness & atrophy
Positive symptoms
• Paresthesia
• HyperalgesiaPeripheral Nerve R
DEMYELINATION
• Common cause of neuropathy, esp. in autoimmune conditions like multiple sclerosis
and Guillain-Barre syndrome
• Glial cells unwind and decrease thickness of myelin, facilitating current leakage
through the membrane
• In demyelinated areas, AP conduction is slowed or halted because excess current
leaks out through non-gated K channels.
• This reduces the available current for the next node of Ranvier, eg.

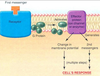
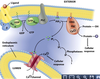
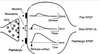
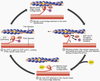
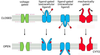
- Compare ionotropic and metabotropic transmission in terms of receptor and postsynaptic actions, duration of effect.
Picture: METABOTROPIC (INDIRECTLY) GATED TRANSMISSION
- Compare ionotropic and metabotropic transmission in terms of receptor and postsynaptic actions, duration of effect.
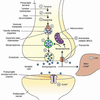
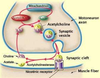
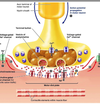
SYNAPTIC TRANSMISSION
• chemical means by which
neurons communicate with
each other
Transmitter release
• Presynaptic membrane
• Synaptic cleft
• Postsynaptic membrane
IONOTROPIC (DIRECTLY) GATED TRANSMISSION
• Receptors: transmitter / ligand gated ion channels
• neurotransmitter increases permeability to ions, here Na.
METABOTROPIC (INDIRECTLY) GATED TRANSMISSION
• Cell signaling systems that affect postsynaptic enzymes
and/or genomic regulation (“metabolism”)
• Receptors
• Membrane receptors: lipophobic transmitters, eg. adrenergic. (focus of this section)
• Intracellular receptors: lipophilic transmitters, eg. steroid (eg. cortisol) and thyroid hormones
Compare ionotropic and metabotropic systems
• Ionotropic receptor = ion channel; fast onset, shorter duration
• Metabotropic receptor = protein that activates signal transduction; slow onset, longer duration
• Eg. tyrosine kinase, part of a receptor, phosphorylates cytoplasmic proteins
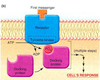
Picture: METABOTROPIC (INDIRECTLY) GATED TRANSMISSION

- Compare ionotropic and metabotropic transmission in terms of receptor and postsynaptic actions, duration of effect.
- Compare ionotropic and metabotropic transmission in terms of receptor and postsynaptic actions, duration of effect. >>Picture: Ionotrophic (Directly) gated transmission
SYNAPTIC TRANSMISSION
• chemical means by which
neurons communicate with
each other
Transmitter release
• Presynaptic membrane
• Synaptic cleft
• Postsynaptic membrane
IONOTROPIC (DIRECTLY) GATED TRANSMISSION
• Receptors: transmitter / ligand gated ion channels
• neurotransmitter increases permeability to ions, here Na.
METABOTROPIC (INDIRECTLY) GATED TRANSMISSION
• Cell signaling systems that affect postsynaptic enzymes
and/or genomic regulation (“metabolism”)
• Receptors
• Membrane receptors: lipophobic transmitters, eg. adrenergic. (focus of this section)
• Intracellular receptors: lipophilic transmitters, eg. steroid (eg. cortisol) and thyroid hormones
Compare ionotropic and metabotropic systems
• Ionotropic receptor = ion channel; fast onset, shorter duration
• Metabotropic receptor = protein that activates signal transduction; slow onset, longer duration
• Eg. tyrosine kinase, part of a receptor, phosphorylates cytoplasmic proteins
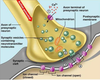
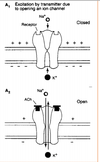
- What are EPSPs in terms of transmitter, receptor, postsynaptic action and impact on the initial segment? Do the same for IPSPs.
- What are EPSPs in terms of transmitter, receptor, postsynaptic action and impact on the initial segment? Do the same for IPSPs. Picture: EPSP!
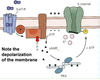
Excitatory Postsynaptic Potentials (EPSP)
• Transient by transmitter-gated Na+/K+ channel
• Na+ influx depolarizes, while K+ exit polarizes,
but net effect is depolarization.
• Excitatory action
• Excitatory synapses (glutamate, eg) usually occur on
dendrites
–
Inhibitory Postsynaptic
Potential (IPSP)
• Transient hyperpolarization by
transmitter-gated Cl- or K+
channel
• Inhibitory action
• Inhibitory synapses (GABA, γ-
aminobutyric acid & glycine)
usually on cell bodies