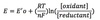Muscle Weakness Module Flashcards
(105 cards)
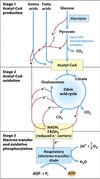
Intermediary Metabolism
All the chemical changes that are involved in the occurance and continuance of life.
Ability to accomplish these changes at constant body temperature requires enzymatic catalysis & thermodynamic coupling of endergonic and exergonic processes.
Pathway
The series of steps involved in the breakdown or synthesis of major biological constituents.
Cycle = pathway that regenerates the initial substrate
Catabolic and anabolic pathways linked via ATP.
Catabolism
Degradative (complex to simpler)
Oxidative
Exergonic
ATP generating
Often requires NAD+ or FAD
Anabolism
Sum of the pathways that are involved in synthesis and growth.
Reductive
Energy consuming
ATP utilization
Often requires NADPH
Metabolic rate
Expression of enthalpic change.
Gives the normalized total heat production per unit time.
Basal metabolic rate
Measured in a resting state (awake laying still)
Energy requirements for:
- involuntary muscle work
- maintenance of osmotic gradients
- maintenance of body temperature
- turnover and synthesis of cell constiuents
Gibb’s free energy
(ΔG)
ΔG = ΔH - TΔS
H= enthalpy S= entropy T= temp in kelvins
The energy change occuring under conditions of constant pressure and temperature.
Additive function and independent of pathway.
Total ΔG of a process can be expressed as the sum of ΔG changes of individual steps.
Thermodynamic Coupling
Coupling of an exergonic and endergonic reaction so that net ΔG is negative and reaction can occur.
A common intermediate must exist.
In an enzyme catalyzed reaction, the common intermediate may not be free and may only exist on the enzyme.
Standard reduction potentials
(Eº)
The relative affinity of a molecule, atom, or ion for electrons taken under standard conditions where reactants and products are at unit activity (~1 M) then compared to the proton/hydrogen electrode (H+ and ½ H2)
Often expressed as values corrected to pH 7 (Eº’)
Interpreted as the relative affinity of the system for electrons as compared to that of a proton:
- Negative reduction potential indicates a weaker affinity for electrons than a proton.
- Positive reduction potential indicates a stronger affinity.
Cellular reduction potential
Dependent on the ratio of oxidant to reductant agent concentration in a cell.
Nernst equation
E = Eº’ + 2.3 RT/nF log [oxidant] / [reductant]
R = gas constant
F = Faraday constant
Relationship of reduction potential to ratio of oxidant to reductant agents in a cell.
High energy bonds
Describes a bond which has a large negative standard Gibb’s free energy (ΔGº’) of hydrolysis
Minimum of -7.0 kcal/mol
Indicated with a ~
Adenosine triphosphate
(ATP)
- Contains two phosphoanhydride bonds which have a ΔGº’ of hydrolysis of approximately -7.3 kcal/mol each.
- Biological utilization of these high energy bonds require the ATP form.
- One or both bonds may be used in a reaction.
- Phosphoryl group(s) of ATP can be transferred to acceptor molecules to generate activated intermediates for metabolism.
- Can function as coenzyme-cosubstrates.
Pyrophosphate
(PPi)
Use of both phosphoanhydride bonds of ATP is the functional equivalent of using 2 ATP’s.
Reactions coupled to the hydrolysis of the pyrophosphate (PPi) product to 2 orthophosphates (Pi) by pyrophosphatase which drives reactions forward.
Adenylate kinase
Catalyzes the reversible reaction:
AMP + ATP ⇔ 2 ADP
Adenine nucleotide cosubstrate metabolic pool
Total concentration of adenine nucleotide pool essentially constant, however, ratio of adenine nucleotides vary with metabolic state of the cell.
Concentrations of ATP, ADP, and AMP in a cell are in rapid equilibrium due to activity of adenylate kinase.
The cosubstrate pool communicates between and significantly influences the different pathways of the cell which utilizes those cosubstrates.
AMP level most sensitive parameter to change in pool and usually initiates the responses to decreased ATP levels.
Energy charge
Energy charge = ½ • ( [ADP] + 2[ATP] ) / ( [AMP] + [ADP] + [ATP])
A stoichiometric expression of the mold fraction of high energy phosphate bonds present relative to the maximal high energy bonds possible.
0 = nucleotides are totally in the form of AMP
1 = nucleotides are totally in the form of ATP
ΔG for ATP hydrolysis
ΔGATP→ADP = ΔGº’ATP→ADP + RT ln ( [ADP] x [Pi] / [ATP] )
Actual ΔG for ATP hydrolysis in a cell is dependent upon and may be calculated from the concentrations of ATP, ADP, and inorganic phosphate.
May be very different from the ΔGº’.
In a resting cell may be as high as = 15 kcal/mol which is equivalent to an energy charge of approximately 0.9.
“High energy charge”, “large negative ΔG of ATP hydrolysis”, and “resting cell” all indicate that ATP is plentiful.
Pathway regulation
- Pathways for synthesis and breakdown of the same constituent are never the same, although individual steps may be reversible and utilized in both pathways.
- Opposing pathways typically occur in seperate cellular compartents and/or different tissues or organs.
- Regulation of pathway enzyme activity by:
- product inhibition
- allosteric regulation
- covalent regulation i.e. phosphorylation
- Changes in the rate of synthesis/degradation of an enzyme or its mRNA.
Equation for glycolysis
glucose + 2 NAD+ + 2 ADP + 2 Pi
→
2 pyruvate + 2 NADH + 2 ATP + 2 H2O + 2 H+
General stages of glycosis
- Priming steps
- Two ATP-linked phosphorylations
- Functions to prevent diffusion of the intermediates of the pathway out of the cell.
- Oxidation/reduction with the production of NADH
* Results in the generation of ATP - Re-oxidation of the NADH produced.
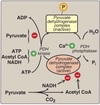
Substrate-level phosphorylation
The synthesis of ATP involving two coupled reactions linked by a common intermediate containing a high-energy bond.
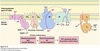
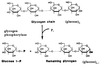
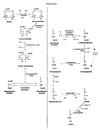
Steps of glycolysis
- Irreversible phosphorylation of glucose at carbon-6 by hexokinase using ATP•Mg2+ to produce glucose-6-phosphate.
- Hepatic isozyme is glucokinase.
- Traps glucose because cell membrane is impermeable to phosphate esters.
- Commits glucose to intracellular metabolism but NOT glycolysis.
- Freely reversible aldose-ketose isomerization of glucose-6-phosphate to fructose-6-phosphate by phosphoglucose isomerase.
- Irreversible phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate using ATP by phosphofructokinase (PFK1).
- True commitment step and primary regulatory site for glycolysis.
-
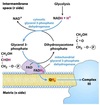
Fructose-1,6-bisphosphate split into two triose phosphates: dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) by aldolase.
- DHAP freely interconverted to GAP by triose phosphate isomerase.
- Only GAP enters next stage of glycolysis.
-
Glyceraldehyde-3-phosphate dehydrogenase catalyzes the reversible oxidation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
- NAD+ converted to NADH **
- Phosphate from the carboxyl group from 1,3-bisphosphoglycerate is reversibly transferred to ADP to produce ATP and 3-phosphoglycerate by 3-phosphoglycerate kinase.
- First substrate-level phosphorylation.
- Additional mutase found in erythrocytes which transfers carbonyl phosphate of 1,3-bisphosphoglycerate to carbon-2 to produce 2,3-bisphosphoglycerate (2,3-BPG).
- 3-phosphoglycerate converted to 2-phosphoglycerate by phosphoglycerate mutase.
- 2-phosphoglycerate converted to phosphoenolpyruvate (PEP) by enolase with loss of H2O.
- Phosphate of PEP is transferred to ADP by pyruvate kinase (PK) to produce pyruvate and ATP.
- Irreversible
- Has a ΔGº’ of -14 kcal/mol
- NADH produced is recycled back to NAD+
- In aerobic conditions via malate shuttle.
- In anerobic conditions pyruvate converted to lactate via lactate dehydrogenase.
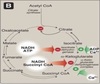
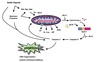
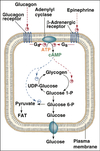
Malate shuttle
Used for the indirect transport of the electrons from glycolysis in the cytoplasm to the respiratory chain in mitochondria.
- Cytosolic oxidation of NADH coupled with reduction of oxaloacetate to malate.
- Malate transported across the inner mitochondrial membrane.
- Malate oxidized back into oxaloacetate as mitochondrial NAD+ is reduced to NADH.