Carbohydrate Metabolism Flashcards
(52 cards)
Tissue Specialization
In multi-cellular organisms:
- Different tissues assume specialized roles and play different roles in metabolism.
- Some may lack one or more of the basic catabolic pathway (ex. brain)
- Some may carry out unique functions and exhibit special pathways and processes (ex. liver)
- Functional differences between tissues frequently reflects a differing regulation of metabolism and/or the occurance of different isozymes.
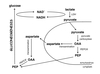
Systemic Coordination
of
Metabolism
Specialized metabolism of different tissues requires regulation and integration by hormones.
Systemically circulated hormones can be used to coordinate the metabolic activities of a wide range of tissues simultaneously.
Most short term responses of the target tissues to hormone binding are the result of regluation of protein phosphorylation.
Three important hormones involved in short-term metabolic regulation are:
- Epinephrine: released from the adrenal medula in response to stress.
- Glucagon: released from the alpha cells of the pancreas in response to low blood glucose and insulin levels.
- Insulin: released from the beta celsl of the pancreas in response to high blood glucose levels.

Glucagon Mechanism
Acts via its G-protein coupled receptors which utilizes cAMP as a second messenger.
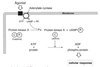
Epinephrine Mechanism
- Can act via a G-protein coupled receptor utilizing cAMP as the secondary messenger.
- Many regulatory pathways act via cAMP and PKA.
- Can act via α-adrenergic receptors (also G-protein coupled) utilizing both diacylglycerol (DAG) and inositol triphosphate (IP3) as secondary messengers.
- Note that after DAG is cleaved from PIP2 it will remain in the plasma membrane.
- Relative number of enzymes affected via the Ca2+/calmodulin protein kinase pathway is less compared to PKA.

Insulin Mechanism
- Functions through a tyrosine-kinase receptor.
- Can also utilize phosphatidylinositol-3,4,5-P3 (PIP3) as a secondary messenger.
- Protein kinase B (serine kinase) is responsible for many of the metabolic effects of insulin including:
- Activation of glycogen synthase by inhibition of glycogen synthase kinase 3.
- Activation of glycolysis in muscle.
- Inhibition of lipolysis in adipose tissue.

Glucose Uptake
- Glucose transported across cell membranes by:
- Facilitated diffusion via GLUT transports down their concentration gradients.
- In renal and intestinal epithelium, transported against its concentration gradient by Na+-glucose co-transporters (SGLTs)
GLUT transporters
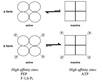
- Glucose-dependent tissues such as RBC’s and brain have low Km insulin-independent GLUT1 or GLUT3 transports respectively.
- In peripheral tissues such as muscle which are glucose-independent, GLUT4 transports have a low KM but is insulin-dependent ⇒ allows cross regulation.
- The liver which does not rely on glucose for energy, uses the GLUT2 transporters have a high KM for glucose but is insulin-independent.
- Limits glucose uptake to conditions when blood glucose levels are high.
- Allows the transporter to act as a sensor of high blood glucose levels.
* Normal fasting blood glucose levels are 3.9 - 5.5 mmol/L.

GLUT 4 Regulation
- Located in muscle and adipose tissue.
- Relatively low Km for glucose so would transport around normal fasting levels.
-
Insulin-dependent glucose transporter:
- When insulin is absent, the transporters are removed from the plasma membrane and sequestered into vesicles.
- Functional but not in the membrane.
- Insulin signaling stimulates the movement of the transporter from internal stores to the plasma membrane.
- When insulin is absent, the transporters are removed from the plasma membrane and sequestered into vesicles.
-
In skeletal muscle, exercise stimulates GLUT4 translocation to the plasma membrane through AMP-activated protein kinase (AMPK) via unknown mechanism.
- Long-term exercise also increases the amount of GLUT4 in the muscle cell.

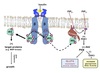
Mechanism of Insulin
GLUT4 Activation
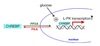
- Binding of insulin to the α-subunit of its receptor activates a tyrosine kinase domain resulting in auto-cross-phosphorylation of tyrosine residues in the β-subunits.
- Negative charge of the phosphates causes IRS (insulin receptor substrate) proteins to bind to the β-subunit.
- IRS proteins phosphorylated at two Tyr residues by the kinase activity of activated insulin receptor.
- Phosphorylated-IRS dissociate from the receptor then bind to and activate proteins with SH2 domains i.e. PI-3-kinase (Phosphatidylinositol-3-kinase).
- PI-3-kinase phosphorylates PIP2 to PIP3.
- PIP3 activates PDK-1 (phosphoinositide-dependent kinase).
- PDK-1 activates downstream effectors Akt and PKB which results in the movement of GLUT4 to the cell surface in adipose and muscle, increasing glucose uptake.
Akt/PKB also:
- phosphorylates and inactivates GSK3 (glycocen synthase kinase 3) resulting in increased glycogenesis.
- Activate amino acid uptake and protein synthesis
- Increase lipid synthesis
- Inhibit gluconeogenesis
- decrease cAMP levels by activating phosphodiesterase
- various gene expression modulations both +/-
- Increases protein synthesis by activating a kinase (mTOR) that ultimately results in the activation of eIF4 and EF2 from protein translation.
Glucose Phosphorylation
- Phosphorylation of glucose prevents back diffuse out of the cell via the transporter and commits it for use in that cell.
-
Hexokinases
- Found in tissues such as muscle and brain.
- Has a low KM for glucose
- Can phosphorylate other monosaccharides but affinity for glucose considerably higher
- Show product inhibition by glucose-6-phosphate.
-
Glucokinase
- Found in liver and pancreatic β-cells
- Has a high KM for glucose
- Shows no direct product inhibition

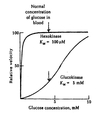
Glucokinase Kinetics
- Despite being monomeric, glucokinase displays sigmoidal kinetics towards glucose.
- The inflection point of the glucokinase enzyme curve is such that small changes in blood glucose levels causes significant changes in enzymatic activity.
- When blood glucose levels are high the hepatic glucokinase becomes significantly more active.
- Hexokinase, however, is fully saturated a normal concentrations of blood glucose.
Glucokinase Regulation
Glucokinase activity is not directly regulated by its product but is indirectly regulated.
- In hepatocytes, glucokinase binds to GKRP (glucokinase regulatory protein) which acts as a competitive inhibitor.
- Glucokinase-GKRP complex is translocated into the nucleus where glucokinase is held in an inactive state.
-
Fructose-6-phosphate strongly stimulates this association.
- F-6-P is in equilibrium with G-6-P
- Fructose-1-phosphate or high glucose concentrations reverse the inhibition by triggering dissociation of the complex.
Transcription of the glucokinase gene is up-regulated in response to insulin ⇒ hormonal control.
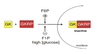
Regulation of glucose utilization
Cells must both take up and then phosphorylate glucose in order to utilize it for metabolic proesses.
Tissue-specific regulation of these two processes alows control of glucose utilization in a manner specific to the needs and function of the tissue.
- Tissues which are glucose-dependent are controlled by product negative feedback only.
- Tissues which are glucose-independent are controlled by blood glucose concentration and product inhibition.
- Glucose-producing tissues are controlled by blood glucose concentrations only.

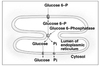
Role of Glucose-6-Phosphate
G-6-P lies at a branch point for several pathways of carbohydrate metabolism.
Its production and utilization by various metabolic pathways are key regulatory points.

Redox Balance
NADH produced during glycolysis must be recycled back into NAD+ for glycolysis to continue.
In aerobic conditions, reduction equivalents of NADH are shuttled into the mitochondria to undergo oxidative phosphorylation.
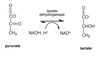
In anerobic conditions, pyruvate is converted to lactate via homolactic fermentation with the regeneration of NAD+.
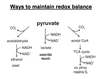
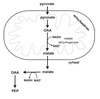
Malate-Aspartate Shuttle
- Electrons are transferred from cytosolic NADH to oxaloacetate forming malate and NAD+.
- Malate enters the mitochondrial inner membrane via malate/α-ketoglutarate transporter.
- Inside the matrix, malate is reoxidized by malate dehydrogenase and NAD+ to form OAA and NADH.
- OAA is converted to aspartate via a transamination reaction.
- Aspartate is transported back to the cytosol via a glutamate/aspartate transporter.
- In the cytosol the aspartate undergoes transamination to reform OAA.
*Shuttle is readily reversible: important in gluconeogenesis.

Glycerol-3-Phosphate Shuttle
Couples the cytosolic oxidation of NADH with the mitochondrial reduction of FAD.
- Cytoplasmic NADH utilized by glycerol-3-phosphate dehydrogenase to convert dihydroxyacetone phosphate to glycerol-3-phosphate.
- Glycerol-3-phosphate is then converted back to DHAP by mitochondrial version of the dehydrogenase which resides on the inner mitochondrial membrane. FAD is reduced to FADH2.
- Electrons from FADH2 are trasferred to the electron carrier Q which enters the respiratory chain as QH2.
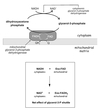
Homolactate Fermentation
Pyruvate converted to lacate under anaerobic conditions.
Concomitant oxidation of NADH to NAD+ restores redox balance in the cytoplasm and enables glycolysis to continue.
Lactate enters the blood and is ultimately reconverted to glucose in the liver via gluconeogenesis.
Excess H+ and lactate inhibits glycolysis.
PFK1
(6-Phosphofructo-1-kinase)
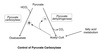
Regulation and Effectors
Most important regulatory step in glycolysis because:
- It is the commitment step for the glycolytic pathway.
- It is allosterically modulated by many metabolic intermediates and products.

Mechanism for the Allosteric Regulation
of
6-phosphofructo-1-kinase
(PFK1)
Allosteric effectors act by influencing the equilibrium between the active and inhibited forms of the enzyme by binding preferentially to one form or the other and stabilizing that form.
-
Fructose-6-Phosphate
- Shows postive cooperativity in binding of PFK1 ⇒ reflected by the sigmoidal shape of [F-6-P] to Vo curve.
-
ATP
- Vo initially rises with increasing [ATP] then falls again.
- ATP acts at two different sites:
- Is a substrate for the reaction
- Is a negative heterotropic allosteric effector ⇒ ATP inhibits F-6-P binding
- Since ATP affects substrate binding and not the Vmax it is K-type regulation.

Role of
Fructose-2,6-Bisphosphate
Fructose-2,6-bisphosphate (F-2,6-P2) is a positive heterotropic effector of PFK1.
F-2,6-P2 is is synthesized and degraded by a multifunctional enzyme with both:
- kinase activity ⇒ PFK2
- phosphatase activity

PFK2
(6-phosphofructo-2-kinase)
Isozymes
&
Regulation by Tissue
Multifunctional enzyme reponsible for synthesis and degradation of fructose-2,6-bisphosphate:
PFK2 & F26Pase
Phosphorylation of either domain inhibits its catalytic activity.
Liver:
PFK2 enzyme a substrate for cAMP-dependent PKA.
Phosphorylation site for liver PFK2 isozyme lies within the kinase domain.
Phosphorylation → inhibits kinase activity & stimulates phosphatase activity.
Heart:
F26Pase enzyme is a substrate for PKB (Akt).
Phosphorylation site for heart PFK2 isozyme lies within the phosphatase domain.
Phosphorylation → inhibits phosphatase activity & stimulates kinase activity.
Skeletal Muscle:
PFK2/F26Pase isozyme has no phosphorylation sites.
Is not covalently regulated.

Hepatic PFK1 Regulation
- Hepatic PFK1 is primarily regulated by the [F-2,6-P2]
- The primary function of F-2,6-P2 in the liver is to make PFK1 sensitive to regulation by glucagon and other hormones.
- The liver does not consume glucose as fuel during times of need–rather it makes glucose for use by other tissues.

Tissue Differences in the control of PFK1
Liver:
low [glucose]blood → high [glucagon] → [cAMP] increases → high [PKA] → PFK-2 inhibited → low [F-2,6-P2] → PFK-1 inhibited → glycolysis decreases
Heart:
Biochem test → flight or flight response → high [epinephrine] → high [PKA] or [PKB/Akt] → PFK-2 activated → high [F-2,6-P2] → PFK-1 activated → glycolysis increases
Skeletal Muscle:
ATP utilization → high [AMP] → PFK-1 activated → glycolysis increases
In a resting cell:
With an energy charge of 0.8-0.9 (ΔGATP = -14 - -15 kcal/mole)
PFK-1 would be strongly inhibited by ATP