Midterm #3 Flashcards
- Endocrine
- Endocrine regulatory molecule
- Exocrine secretion
*
- endocrine
- “internal secretion”
- endocrine regulatory molecule
- hormone
- a substance that is released into the internal environment of the body (extracellular fluid (ECF))
- Exocrine secretion
- released to external environment
- digestive tract secretion
- stomach, pancreas, and small intestine secrete substances such as digestive enzymes that wind up in the lumen of the digestive tract
- digestive tract is continuous with the external environment, this is exocrine secretion
secretion in endocrinology
whatever occurs to increase the amount of hormone in the circulation
Two broad categories of hormones
polar hormones and nonpolar hormones
Polar hormones
- catecholamines
- dopamine, norepinephrine, and epinephrine
- synthesized in the cytosol through enzymatic modification of the amino acid tyrosine
- transporter protein is responsible for their delivery into secretory vesicles
- peptide hormones
- synthesized in rough ER
- first synthesized as part of a larger preprohormone
- first step is cleavage of the **signal sequence **in the rough ER to form a prohormone
- further processed in the Golgi and secretory vesicles to give rise to the active hormone
- prohormone may give rise to more than one active hormone
- example is pro-opiomelanocortin (POMC)
- both adrenocorticotropic hormone (ACTH) and alpha-melanocyte-stimulating hormone (alpha-MSH)
storage, secretion, and action of the polar hormones
- Hormone receptors in the target cell activate signal transduction pathways that alter cellular activity
- Many polar hormones signal via G-protein coupled receptors (seven transmembrane domain proteins)

Nonpolar steroid examples
- steroid hormones, vitamin D, and thyroid hormones
- steroid and vitamin D through modification of **cholesterol **
- Thyroid hormones are initially synthesized as part of a large protein precursor called thyroglobulin. Tyrosine residues within thyroglobulin are iodinated, and then the hormones are released through proteolysis of thyroglobulin.
Nonpolar hormone secretion, MOA
- lipophilic, nonpolar hormones cannot be stored in secretion vesicles
- regulated by regulating hormone synthesis
- tropic hormone, usually a peptide hormone, whose binding to a receptor on the endocrine cell regulates hormone synthesis
- As hormone is produced, it diffuses across the plasma membrane.
- Hormones in circulation bound to carrier proteins
- albumin, hormone binding proteins
- small amount as free hormone
- can diffuse across plasma membrane of target cell
- Once inside signal by binding to intracellular receptors (nuclear receptors) that bind to DNA
- acts as transcription factor
- nonpolar hormones are often chemically modified by enzymes in target tissues
- T4 (thyroxine) is converted to T3 in tissues through the action of the enzyme deiodinase

tropic hormones
- hormone that stimulates hormone secretion
- also stimulate proliferation of endocrine cells
Regulation of Nonpolar Hormone Secretion: Examples
- tropic hormone stimulates hormone synthesis in the endocrine cell
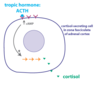
- Ex: adrenocorticotropic hormone (ACTH) stimulates secretion of cortisol in zona fasciculata of the adrenal cortex.
- ACTH secreted by adenohypophysis
- via circulation to zona fasciculata, binds G-protein coupled receptor (7 transmembrane domain protein).
- stimulates adenylyl cyclase and prodction of cAMP
- activates cortisol synthesis enzymes
- peptide hormones that stimulate nonpolar hormone release are the gonadotropins (FSH and LH), and thyroid stimuating hormone (TSH)
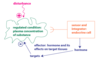
Regulation of Polar Hormone Secretion (Ex: GH)
- tropic hormones stimulate cell secretion (exocytosis)
- tropic hormone growth hormone releasing hormone (GHRH)
-
hypophysiotropic hormone
- Sole purpose to regulate hormone release in adenohypophysis
- produced in hypothalamus and released at capillary bed called median eminence
- Go directly to adenohypophysis via hypophyseal portal vessels
-
hypophysiotropic hormone
- GHRH is a GCPR, increasing cAMP, excytosis of vessicles containing peptide hormone GH

hormones can inhibit hormone secretion
- somatostatin
- hypothalmic hormone inhibiting GH secretion
- Negative feedback regulation
Humoral Regulation
- humoral: fluid of the body (ECF, including blood plasma)
- humorally regulated hormones, endocrine cells regulated by concentration in ECF
-
homeostatic regulation
- stabalize and maintain
- forms negative feedback loop
-
homeostatic regulation
- minus sign indicates that opposing disturbance
PTH Secretion
- parathyroid hormone (PTH)
- humorally regulated
- secreted by parathyroid glands
- functions in calcium homeostasis
- ECF in tight range because of stability effect on voltage-gated ion channels
- calcium too low, open spotaneously, muscle spasms called hypocalcemic tetany
- stimulus for PTH secretion is hypocalcemia
- stimulates release of calcium from bone by stimulating bone resorption
- decreases the amount of calcium excreted in the urine, by stimulating calcium reabsorption in the kidney
- indirectly promotes calcium absorption by the digestive tract
- activates the enzyme in kidney cells that produces the hormone 1,25-(OH)2D (the active form of vitamin D)
- sensor on parathyroid gland that detects calcium levels is calcium receptor (GCPR)
- calcium high, binds receptor and inhibit PTH secretion
- low calcium, receptor unbound
-
calcimimetics
- drugs that mimic calcium at calcium receptor
-
cinacalcet
- treat hyperparathyroidism

Insulin Secretion
- humorally regulated
- shift the cells in the body into the absorptive state
- after meal, body taking up and using glucose, making storage molecules glycogen and triacylglycerol
- Glycogen: skeletal muscle and liver
- Triacylglycerol: liver and adipocytes
- liver packages triacylglycerol in particles of very low density lipoprotein (VLDL) for export to adipose tissue.
- stimulator of insulin secretion, concentration of glucose in the plasma
- pancreatic beta cell senses plasma glucose
- enters the cell by a glucose transporter
- metabolism of gluose to ATP,
- ATP binds and closes ligand-gated K+ channel
- depolarization of cell
- opens voltage gated calcium channels
- calcium enters cell and triggers exocytosis of secretory vesicles containing insulin

Potassium Channel as drug target for T2DM
- insulin in blood normal, but unreceptive so hyperglycemia ensues
-
Sulfonylureas and meglitinides
- increase insulin secretion
- binding to and closing the potassium channels
- Not work in T1DM because autoimmune disorder where beta cells are destroyed
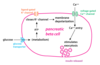
Incretins: Overview
- increase insulin secretion
- more insulin with oral vs. IV glucose
- hypothesis: glucose activated feedforward mechanism to release glucose

two main incretin hormones in humans
- GIP (glucose-dependent insulinotropic peptide also known as gastric inhibitory peptide)
- GLP-1 (glucagon-like peptide-1)
- From endocrine cells located in epithelium of small intestine
- increase of glucose in digestive tract trigger incretin release
- via circulation to pancreatic beta cells
- stimulate pancreatic beta cells to cause insulin release

several reasons why treatments with an incretin analogue, particularly a GLP-1 analogue, could be really beneficial for T2DM
- Defective incretin action in T2DM
- many T2DM insufficient insulin after meals
- less GLP-1 secretion and beta cells less responsive to GIP
- Glucose-dependent effect on insulin secretion
- incretins are glucose-dependent
- incretins augment glucose-stimulated insulin secretin
- T2DM drugs that increase insulin secretion may cause hypoglycemia, because insulin regardless of blood glucose levels
- incretin drugs wouldn’t
- Other effects of GLP-1 beneficial for the treatment of T2DM
- inhibits glucagon secretion
- glucagon works oppositely to insulin, stimulate glucose production inliver
- GLP-1 delays stomach emptying, spread glucose absorption overtime
- lose weight because delayed stomach emptying
- increase the number of beta cells
- inhibits glucagon secretion
new incretin-based drugs have been developed and approved for the treatment of T2DM
- used in conjunction with other anti-diabetic drugs for T2DM
- GLP-1 analogs are GLP-1 receptor agonists
- more stable than the native peptide, and resistant to degradation by DPP-4, the main protease that breaks down GIP and GLP-1
- DPP-4 inhibitors
- “gliptins”
- prolong the action of the native incretins by preventing their breakdown
- less effective in promoting glycemic control as the GLP-1 analogs, an advantage of the DPP-4 inhibitors is that they can be taken orally
Autonomic Inervation of the Pancreas
- preganglionic neuron has cell body in CN
- postganglionic neuron has cell body in autonomic ganglion
- Parasympathetic input
- stimulates insulin secretion
-
cephalic phase stimulation of insulin secretion
- Sensory stimuli and neural inputs activated when food is first eaten
- activation of parasympathetic preganglionic neurons whose axons travel in the vagus nerve
- activate postganglionic neurons that stimulate insulin secretion even before there is an increase in blood glucose
- feedforward (release in anticipation)
- Sympathetic input
- inhibits insulin secretion
- important during exercise
- need to activate fuel-burning mechanisms and prevent glucose uptake by non-muscle cells
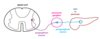
Adrenal Medulla
- adrenal medulla is considered a modified sympathetic ganglion
- innervated by sympathetic preganglionic neurons
- adrenal medulla release norepinephrine (but they also release epinephrine)
- considered hormones b/c released into circulation
- bind to adrenergic receptors, and thus have much the same physiological effects as sympathetic neural stimulation
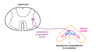
Neurosecretory Cells
- endocrine cells in brain
- terminals adjacent to capillaries
- secrete molecules that enter circulation and act as hormones
- secretion regulated by hormones that neurons that form synapses with the dendrites of neurosecretory cells
- Neurosecretory cells are found in the hypothalamus
- regulates necessary life processes
*
- regulates necessary life processes

two types of neurosecretory cells
- Magnocellular cells
- larger, having longer axons that terminate in the neurohypophysis
- parvocellular cells
- smaller, with shorter axons that terminate at a capillary-rich bulge at the base of the hypothalamus known as the median eminence
communication between the hypothalamus and adenohypophysis
- parvocellular cells release tiny amounts of various hormones whose sole function is to regulate hormone release by endocrine cells in the adenohypophysis
- hormones to adenohypophysis via hypophyseal portal vessels

hypothalamus and pituitary: negative feedback
- hormone from peripheral gland (ex: cortisol) bind in pituitary and hypothalamus and inhibit tropic hormone secretion (ex: CRH (corticotropin releasing hormone) and ACTH (adrenocorticotropic hormone))
- Less CRH leads to less ACTH which leads to less cortisol secretion (in zona fasiculata of adrenal cortex)
- “hormonal homeostasis”
*

“Cushing’s Syndrome”
- hypersecretion of cortisol
- central obesity, insulin resistance, bone reabsorption (osteoporosis), and hypertension
- tumor in adrenal cortex
- increased negative feedback from cortisol
- ACTH is low
*

Cushings Disease
- cortisol hypersecretion
- central obesity, insulin resistance, bone reabsorption (osteoporosis), and hypertension
- pituitary adenoma
- ACTH and cortisol levels high

Primary adrenal insufficiency
- hyposecretion of cortisol
- problem originates in adrenal gland
- damage to adrenal cortex, hyposecretion of aldosterone (regulate K and Na in ECF)
- hyposecretion of coritsol AND aldosterone is Addison’s Disease
- high levels of ACTH

Secondary adrenal insufficiency
- hypopituitary adrenal insufficiency
- low ACTH levels lead to hyposecretion of cortisol
- can occur after the end of high dose glucocorticoid therapy

what and how glucocorticoids cause hypopituitary adrenal insufficiency
- glucocorticoid is the name for a molecule that is a glucocorticoid receptor agonist; the glucocorticoid receptor is the receptor for cortisol
- Chronic high levels of glucocorticoids lead to a suppression of ACTH secretion
- glucocorticoid therapy is discontinued, the result is hypocortisolism
- avoid with alternate day dosing or taper off

Figure for Cushing’s Syndrome
- First establish hypercortisolism
- roughly circadian pattern in which cortisol secretion is at its lowest around midnight, thus cortisol levels are measured at this time
- level of ACTH is used to distinguish between primary hypercortisolism and Cushing’s disease due to a pituitary adenoma

Figure for Cushing’s Disease

Figure for Addison’s Disease
- Primary adrenal insufficiency
- autoimmune cause, but can also result from tuberculosis
- affect both the zona glomerulosa and the zona fasciculata, so there is hyposecretion of both cortisol and aldosterone
- increased ACTH secretion
- challenge test to diagnose:
- determines the ability of the adrenal gland to respond to exogenously administered ACTH with increased cortisol secretion

Figure for hypopituitary adrenal insufficiency
- Secondary adrenal insufficiency
- consequence of medical therapy with glucocorticoids

How does hyperglycemia cause excessive urine production?
- function kidney unit: nephron
- filtration
- bulkflow of water and small molecules into Bowman’s capsule
- glucose, amino acids, and certain ions end up in the forming urine
- cells lining the kidney tubules transfer these substances out of the forming urine and back into the extracellular fluid. This process is known as reabsorption
- normal circumstances, 100% of the glucose that is filtered is reabsorbed
- Glucose reabsorption involves transport proteins that require specific binding
- diabetes, transporter becomes saturated
- glucose draws water via osmosis

new drugs are being developed that exploit glucose loss in the urine as a means to counteract hyperglycemia
- SGLT2 inhibitors
- inhibiting the sodium-glucose cotransporter (SGLT2) that is specific to the kidney tubules and responsible for 90% of glucose reabsorption
- reduce hyperglycemia and promote weight loss
- increase urine flow, but not to bothersome point
- independent of insulin, can be used in combo with other drugs
- “-gliflozin”
- Canagliflozin (tradename: Invokana)
- Dapagliflozin (tradename: Forxiga)
- Empagliflozin (tradename: Jardiance)
HbA1c test
- measures the percentage of glycosylated hemoglobin
- will be higher if there have been more periods of hyperglycemia in the recent past
- HbA1c of 6.5% or greater is diagnostic for diabetes mellitus
- no fasting, can be done at any time of day
fasting plasma glucose
- morning when not eaten for 8 hours
*
oral glucose tolerance test
- how body responds to glucose
- 75 grams of glucose
- Reflects two things:
- ability of the beta cells to secrete insulin
- responsiveness of cells in the body to insulin
- Diabetes indicated by:
- fasting plasma glucose above 126 mg/dL
- a 2-hour reading greater than 200 mg/dL

at increased risk for the development of type 2 diabetes
- impaired glucose homeostasis
- fasting glucose that is greater than 100 mg/dL is categorized as having impaired fasting glucose
- impaired glucose tolerance, which involves a 2-hour reading of greater than 140 mg/dL

In someone in which type 2 diabetes mellitus is diagnosed, insulin resistance is usually accompanied by a defect in…
- insulin secretion
- initially compensate by secreting more insulin, Hyperinsulinemia
- relative insulin deficiency when the beta cells can no longer produce enough insulin to compensate
Causes of Insulin Resistance: Increased fatty acids and ectopic lipid
- more visceral adipose tissue
- higher rates of lipolysis in visceral adipocytes, resulting in higher levels of circulating fatty acids
- higher rates of lipolysis in visceral adipocytes, resulting in higher levels of circulating fatty acids
- ectopic lipid interefers with insulin sensitivity
- Reduced insulin signal transduction: Fatty acids activate serine-threonine kinases that phosphorylate insulin receptor substrates on different amino acids, inhibiting tyrosine phosphorylation by the insulin receptor. Insulin receptor is a tyrosine kinase
- **Altered metabolism: **Fatty acids stimulate gluconeogenesis in the liver. Thus hepatic glucose production, which should be inhibited by insulin, persists.
- **Increased inflammation: **saturated fatty acids stimulate the innate immune system
Causes of insulin resistance: Changes in adipocyte regulatory molecules
- Adiopoctes secrete regulatory molecules known as adipokines.
- Inflammatory paracrines: TNF-alpha and IL-6, interfering with insulin signal transduction, promoting inflammation in blood vessels
- **Decreased adiponectin: **secretion is decreased in obesity, increases insulin sensitivity and improves metabolism by stimulating fatty acid oxidation
- **Increased cortisol: **post-absorptive state hormones that decrease responsiveness to insulin, Adipocytes have an enzyme that converts an inactive cortisol metabolite back into active cortisol
*
“Apple” shape vs. “Pear” shape
- venous blood that drains visceral adipose tissue in the mesenteries flows first to the liver via the hepatic portal vein, before returning to the heart
- changes in regulatory molecules caused by visceral adipocytes are in a position to directly affect the liver, a key target of insulin.
Causes of Insulin Resistance: Increased inflammation
- obesity is associated with a state of chronic low-level inflammation
- higher number of macrophages
- release inflammatory paracrines that cause insulin resistance.
Improving insulin sensitivity: Diet
- Weight loss improves insulin sensitivity
- negative feedback loop involving the hormone leptin which works to maintain body weight at a set point
- obese individuals, this negative feedback loop works to maintain body weight at a higher than normal set point
- lose weight, lose leptin, hunger and lower energy expenditure
Improving insulin sensitivity: Bariatric Surgery
- Roux-en-Y gastric bypass: smaller stomach, limit nutrient absorption through bypass of the initial part of the small intestine (malabsorption)
- weight loss and insulin resitance resolution
- reorganization of the digestive tract somehow alters the secretion of regulatory molecules that influence insulin sensitivity
Improving Insulin Sensitivity: Exercise
- promotes glucose uptake in skeletal muscle
- promotes the movement of glucose transporters to the muscle cell plasma membrane, and it does so independently of insulin
- weight loss
Improve Insulin Sensitivity: Metformin
- MOA not completly understood
- affects mitochondrial function in hepatocytes.
- outcome of these effects in liver cells is the inhibition of gluconeogenesis
Improve Insulin Sensitivity: Thiazolidinediones (TZD’s; Glitazones)
- TZD’s: agonists for the intracellular receptor known as PPAR-gamma
- expressed in adipocytes and pre-adipocytes
- transcription factor, stimulating adipocyte differentiation andlipogenesis (triacylglycerol formation) in adipocytes
- large, hypertrophic adipocytes are the most problematic), and in part on the increased fatty acids in the circulation
- TZD’s stimulate the formation of greater numbers of small adipocytes, and stimulate apoptosis in hypertrophic adipocytes
- promote fat storage in adipocytes, thus decreasing the level of circulating fatty acids and preventing the pathological effects of lipid accumulation in other tissues (ectopic lipid)
- adipocytes to increase expression of adiponectin, which promotes insulin sensitivity
- Use restricted because of safety concerns
Goal of Treatments for DM, DM Complications
- glycemic control, that is the prevention of hyperglycemia
- Chronic hyperglycemia leads toabnormal glycoslyation
- Diabetic Complications:
- Cardiovascular Disease
- Nephropathy
- Retinopathy
- Peripheral neuropathy
- Foot ulcers
- adverse effect of more intensive diabetic therapy is hypoglycemia
- Glycemic control monitored through HbA1c, or thepercentage of glycosylated hemoglobin
- detects glycemic control in the previous 4-8 weeks
- recommends an HbA1c target of less than 7% for most diabetics
Insulin
- necessary for type 1 diabetics
- absolute insulin deficiency due to the autoimmune destruction of the pancreatic beta cells
- also in T2DM
- later on, beta cells damaged by hyperglycemia
*
- later on, beta cells damaged by hyperglycemia
Sulfonylureas and Meglitinides
- increase insulin secretion
- bind to and block the ATP-sensitive K+ channel on pancreatic beta cells
- depolarization
- cause weight gain
- Sulfonylureas (glyburide, tolbutamide) are older drugs and less expensive
- Risk of hypoglycemia
- meglitinides (repaglinide, nateglinide) are newer drugs that are designed to avoid this problem
- shorter half-life
- taken at mealtimes to enhance insulin secretion and prevent postprandial hyperglycemia.
Incretin-Based Therapies
- increase insulin secretion
- GLP-1 agonists are peptide drugs with a longer half-life than the native hormone because they are resistant to digestion by the protease DPP-4
- DPP-4 inhibitors (“gliptins”) prolong the action of native incretins
- less effective at lowering HbA1c than GLP-1 agonists, but an advantage is that they are oral drugs.
- GLP-1 agonists induce weight loss
- delays stomach emptying
- feel full sooner
Metformin
- insulin sensitizers
- first drug of choice used to treat newly diagnosed diabetes
- pre-diabetics
- liver, where metformin inhibits gluconeogenesis.
Thiazolidinediones
- Insulin sensisitzer
- pioglitazone, rosiglitazone
- agonists for an intracellular receptor known as PPAR-gamma
- expressed in adipocytes,
- affect adipocyte gene expression, ultimately causing decreases in circulating fatty acids
- affect adipocyte secretion of regulatory molecules
- adipocytes secrete more adiponectin, which increases insulin sensitivity, and fewer adipokines that cause insulin resistance.
- Safety concerns
- rosiglitazone (trade name: Avandia) to increased risk of heart attack
- pioglitazone (trade name: Actos)
- weight gain and increased fluid retention, and increase the risk for heart failure
- may be possible to develop PPAR-gamma receptor modulators. The hope is to develop drugs that are insulin-sensitizing without causing adverse effects that increase the risk for cardiovascular disease
Drugs that Decrease Glucagon Secretion
- Glucagon is secreted by the alpha cells in the pancreatic islets of Langerhans
- post-absorbative hormones
- stimulate hepatic glucose production
- Glucagon secretion can be higher than normal, particularly in type 1 diabetes mellitus
- GLP-1 agonists
- pramlintide, a synthetic analogue of the peptide hormone amylin, which is produced by the pancreatic beta cells
- inhibits glucagon secretion and also slows stomach emptying
- adjunct drug
Drugs that Decrease Glucose Reabsorption
- SGLT2 inhibitors
- improve glycemic control and cause weight loss
- canagliflozin, dapaglifolozin, and empagliflozin
- ower blood glucose through a mechanism that is independent of insulin, so they could be safely combined with other treatments.
Diabetes drugs: categorized on affect on body weight

Diabetes drugs categorized on: route of administration

Diabetes drugs categorized on: place in therapy

Leptin and Body Weight Regulation
- Leptin participates in a negative feedback loop that regulates the amount of energy stored in adipose tissue (adiposity), which is a major determinant of body weight
- Leptin is transported into the brain, primarily acting on neurons in the hypothalamus
- decreased energy intake (feeding)
- NOT a satiety signal
- increases metabolic rate

Leptin and obese mutant mouse
- homozygous mutant mice completely lack leptin
- eat constantly, obese , DM from insulin resistance, infertile
- leptin injections cause weightloss
- human obesity NOT from leptin deficiency
- leptin only cause weight loss at high doses
- may be leptin resistant
leptin negative feedback loop may be more important for preventing starvation rather than overfeeding
- scarcity of food more evolutionarily important
- decreased leptin increases hunger and decreases metabolic rate
- leptin administration after weightloss to maintain weight loss

Leptin has been used to treat lipodystrophy
- degeneration of adipose tissue and insulin resistance
- lipodystrophy results in decreased leptin secretion because there is decreased adipose tissue.
- Leptin treatment improves insulin sensitivity in patients with lipodystrophy.
Iodine Deficiency
- Thyroid hormone has a crucial role in the development of the nervous system, being involved in the growth of synapses and the formation of myelin
-
Endemic cretinism is a disorder of cognitive development with reduced physical growth that occurs if thyroid hormone is deficient during gestation and early post-natal life
- making sure that pregnant women have sufficient iodine in their diet to be euthyroid (having adequate thyroid hormone levels)
-
Goiter
- low level of iodine in the diet, then less active T3 and T4 can be synthesized
- reduced negative feedback inhibition of secretion of the tropic hormones, TRH (thyro ropin releasing hormone; released by the hypothalamus) and TSH (thyroid stimulating hormone or thyrotropin; released by the anterior pituitary)
- TSH stimulates proliferation of follicle cells
- individuals may have goiter and yet be euthyroid, because the enlarged thyroid gland is better able to use the limited amount of iodine available

Graves Disease
- most common cause of hyperthyroidism
- agonist antibodies are produced that bind to the TSH receptor
- synthesis of T3 and T4, and proliferation of follicle cells
- levels of TRH and TSH decrease, but this does not decrease thyroid hormone production because the stimulation of the thyroid gland is independent of TSH
- Symptoms:
- heat intolerant
- tachycardia (fast heart rate)
- thyroid hormone affects expression of cardiac ion channels and contractile proteins so that the force and rate of the heart beat are both increased
- thyroid hormone increases the responsiveness to epinephrine and norepinephrin
- nervousness, sweating and increased heart rate
- beta-adrenergic antagonists may be used to relieve symptoms.
- removed surgically (thyroidectomy)
- drugs that inhibit thyroid hormone synthesis
- administer radioactive iodine
*

Hashimoto’s Thyroiditis
- most common cause of hypothyroidism in the United States
- autoimmune destruction of the thyroid gland
- antibodies to thyroid antigens, as well as infiltration by cytotoxic T cells lead to destruction
- may initially develop goiter, (which occurs due to inflammation), rather than symptoms due to hypothyroidism
- thyroid gland stores large amounts of thyroid hormone as thyroglobulin
- As the store of thyroid hormone decreases, negative feedback inhibition decreases and TSH levels will rise
- Symptoms:
- decreased metabolic rate
- gain weight
- feel sluggish and cold
- slowed heart rate
- replacement therapy with thyroxine (T4).

At the cellular level growth can occur either through
- proliferation (hyperplasia)
- increase in cell size (hypertrophy)
- cell size is limited by limitations in the distance of diffusion, so most growth occurs by an increase in cell number
Proper growth is a function of
- adequate nutrition
- appropriate endocrine regulation
- natural measure of the growth that occurs during childhood is the eventual height attained, or stature
- function of growth by the long bones in the body,
*
- function of growth by the long bones in the body,
Bone Growth
- long bones form by a process that is known as endochondral ossification
- mesenchymal cells condense and then differentiate as chondrocytes to form a cartilage model of the bone
- Mesenchymal cells are the developmental precursors of connective tissue cells
- cartilage model is replaced by bone
- first, shaft is ossified
- chondrocytes enlarge and change, and blood vessels penetrate into the tissue, carrying bone precursor cells (osteoblasts) that secrete bone matrix
- Ossification of the shaft spreads outwards towards the epiphyses (ends of bone)
- Secondary ossification centers eventually appear in the epiphyses
- all cartilage replaced by bone except for at epiphyseal growth plate.
- mesenchymal cells condense and then differentiate as chondrocytes to form a cartilage model of the bone

Postnatal growth
- stimulation of chondrocyte proliferation at the epiphyseal growth plates
- keep growing if cartilage proliferation keeps pace with the rate of ossification
- gonadal steroids (estrogen, testosterone) cause closure of the epiphyseal growth plates
Endocrine Regulation of Growth
- key endocrine regulator of postnatal growth is growth hormone (GH)
- secreted by cells in the adenohypophysis
- GH indirectly stimulates growth at the epiphyseal plate
- GH stimulates production of insulin-like growth factor-1 (IGF-1)
- IGF-1 is hormone and paracrine
- some in liver and to bone via circulation
- some produced locally by chondrocyte cell
- IGF-1 tdirectly stimulates chondrocyte cell division and bone growth
- Regulation of GH secretion
- hormones that are secreted by parvocellular cells in the hypothalamus
- both a stimulatory hormone,growth hormone releasing hormone (GHRH) and an inhibitory hormone, somatostatin
- hormones that are secreted by parvocellular cells in the hypothalamus
- Thyroid hormone is important for growth because it promotes growth hormone synthesis.
- Gonadal steroids (estrogen and testosterone), whose secretion increases at puberty, initially promote growth by increasing GH secretion, and then subsequently cause growth to end by causing the closure of the epiphyseal growth plates.
- Cortisol, which is released in response to stress, causes an inhibition of growth.
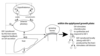
Growth Disorders
- Short stature
- defect in GH secretion (growth hormone deficiency)
- treated with GH replacement
- defect in the growth hormone receptor (growth hormone resistance)
- Laron dwarfism
- treated with IGF-1 replacement
- defect in GH secretion (growth hormone deficiency)
- Hypersecretion of GH by a pituitary tumor
- Childhood: gigantism, rare
- Adulthood: acromegaly
- insidious onset b/c of slow growing tumor
- High levels of GH and IGF-1 cause excessive growth of soft tissues and appositional growth in certain bones, particularly the jaw and skull, leading to disfiguring facial changes
- insulin resistance and lipid disorders
- heart disease and arthritis
- Treated with surgery to remove tumor
- somatostatin agonist, such as octreotide
Calcium Homeostasis
- regulation of the concentration of calcium ions in the extracellular fluid [Ca++]ECF
- stabilizing effect on voltage-gated ion channels
- (hypocalcemia), voltage-gated ion channels start opening spontaneously
- nerve and muscle cells to become hyperactive
- muscle spasms, hypocalcemic tetany
- (hypercalcemia), voltage-gated ion channels don’t open as easily
- depressed nervous system function
- deposits of calcium phosphate (stones) in blood vessels and in the kidneys.
Endocrine Regulation of [Ca++]ECF
- parathyroid hormone (PTH)
- major regulator
- secretion is stimulated by hypocalcemia
- stimulates the release of Ca++ from bone, in part by stimulating bone resorption.
- decreases urinary loss of Ca++ by stimulating Ca++ reabsorption
- indirectly stimulates Ca++ absorption in the small intestine by stimulating synthesis of 1,25(OH)2D in the kidney
- 1,25(OH)2D (the active form of vitamin D)
*

PTH Effects on Bone
- rapid effect
- stimulates osteoblasts to pump Ca++ ions out of the fluid surrounding the bone (which has a higher Ca++ concentration) and into the ECF
- long term effect
- stimulates bone resorption
- osteoblasts that express PTH receptors
- PTH stimulation of osteoblasts causes them to express a signaling molecule that activates osteoclasts
PTH Effects on Kidney
- decreases the loss of Ca++ ions in the urine by stimulating Ca++ reabsorption
- also inhibits phosphate reabsorption in the kidney.
- stimulate production of 1,25(OH)2D
- A precursor (known specifically as vitamin D3 or cholecalciferol) is synthesized in a photochemical reaction in the skin, in response to sunlight
- chemically modified in the liver to form 25-(OH)D.
- enzyme in the liver is constitutively active, meaning it is always working
- kidney enzyme is regulated
- PTH is to stimulate the regulated kidney enzyme
- resulting in the production of 1,25(OH)2D.
- chemically modified in the liver to form 25-(OH)D.
- A precursor (known specifically as vitamin D3 or cholecalciferol) is synthesized in a photochemical reaction in the skin, in response to sunlight
- 1,25(OH)2D works in the small intestine to promote Ca++ absorption
- kidney disease, inadequate amounts of 1,25(OH)2D
- Ca++ homeostasis is maintained at the expense of bone
- [Ca++]ECF drops because of a lack of Ca++ absorption from the diet
- stimulates high levels of PTH secretion
- secondary hyperparathyroidismbecause the problem that causes the hyperparathyroidism is in the kidney
- treated by administering vitamin D and Ca++ supplements
- cinacalcet, calcimimetic drug that binds to the Ca++ receptor on cells in the parathyroid gland, inhibiting the secretion of PTH.

Bone cells
- Bone remodeling is triggered by a need for calcium in the extracellular fluid
- mechanical stresses as well
- Osteoblasts
- bone-forming cells
- onnective tissue cells found at the surface of bone
- can be stimulated to proliferate and differentiate as osteocytes
- Osteocytes
- bone cells
- manufacture type I collagen and other substances that make up the bone extracellular matrix
- found enclosed in bone.
- Osteoclasts
- bone-resorbing cells
- large, multinucleate cells that form through the fusion of precursor cells
- Unlike osteoblasts, which are related to fibroblasts and other connective tissue cells, osteoclasts are descended from stem cells in the bone marrow that also give rise to monocytes.

Bone resorption
- can be triggered by parathyroid hormone (PTH) in response to hypocalcemia
- PTH stimulates the generation of new osteoclasts (osteoclastogenesis)
- mature osteoclast needs to tightly adhere to the bone, creating a specialized isolated compartment
- membrane adjacent to the bone differentiates as the ruffled membrane
- contains proteins that acidify the compartment adjacent to the bone
- acid dissolves the minerals in the bone; subsequently, digestive enzymes break down type I collagen and other proteins
- Bone resorption ends when the osteoclast dies by apoptosis.
Osteoclastogenesis
- Receptors for PTH are located on osteoblasts, which then signal to bone marrow-derived osteoclast precursors to stimulate their fusion, differentiation and activation
- Osteoclast precursors express a cell-surface receptor known as RANK
- Osteoblasts express RANKL
- stimulated by PTH, osteoblasts up-regulate expression of RANKL, which binds to RANK, activating signaling pathways that promote osteoclast differentiation and survival.
- Osteoblasts also express a secreted factor called osteoprotegerin
- “protects bone” by preventing bone resorption
- decoy receptor for RANKL: it binds RANKL and therefore prevents binding to RANK and stimulation of osteoclastogenesis
- ratio of osteoprotegerin:RANKL produced by osteoblasts will determine the extent of bone resorption.
- Denosumab
- monoclonal antibody that binds to RANKL
- treatment of osteoporosis

Osteoporosis
- severe reduction in bone mass that substantially increases the risk of bone fracture
- Partial reduction of bone mass is referred to as osteopenia.
- Someone with a low peak bone mass is more likely to develop osteoporosis later in life
- peak in 30’s
- genetics, nutrtion, exercise
- calcium, vitamin D, and exercise in bone building years
- Hypersecretion of PTH, thyroid hormone, and cortisol all promote bone loss
- gonadal steroids (estrogen and testosterone) have a key role in maintaining skeletal mass
- suppressing the production of signals that promote osteoclastogenesis
Treatments for Osteoporosis
- treatments for osteoporosis areantiresorptive
- inhibit osteoclasts
- reduce bone loss, but they do not work to build new bone
*
HRT and osteoporosis
- used to be mainstay of treatment
- greater risk of adverse cardiovascular events, as well as an increased risk of breast cancer
*
Bisphosphonates
- first line of therapy for osteoporosis
- non-hormonal drug therapy
- related to pyrophosphate
- inhibiting osteoclast activity and inducing osteoclast apoptosis
- alendronate and risedronate.
*
SERM
- Selective Estrogen Receptor Modulator
- bind to estrogen receptors, acting as estrogen agonists in some tissues, while acting as estrogen antagonists in other tissues
- avoid the adverse effects of administering estrogen, while at the same time gaining the beneficial effect of estrogen on bone health
- raloxifene
Denosumab
monoclonal antibody that binds to RANKL to inhibit osteoclastogenesis. Denosumab mimics the effect of the endogenous protein osteoprotegerin.
Teriparatide
- peptide analog of PTH
- only FDA-approved treatment for osteoporosis that is anabolic
- stimulates osteoblasts to build new bone
- intermittent treatment with PTH actually stimulates bone deposition
- promoting osteoblast survival, or by stimulating osteoblast proliferation
- present chronically, PTH causes bone resorption
- once-daily injection
Calcitonin
- acts oppositely to PTH
- osteoporosis treatment
- calcitonin secretion is stimulated by hypercalcemia and it acts to reduce calcium in the ECF
- binds to receptors on the osteoclast and inhibits bone resorption
- peptide hormone
- injection or nasal spray


