Midterm #1 Flashcards
(148 cards)
1
Q
Primary Structure
A
- Linear sequence of amino acids
2
Q
Peptide Bonds
- Functions
- Picture
A
- Holds together adjacent amino acids in polypeptide chain
- Polar nature around peptide bond

3
Q
Secondary Structures
- 2 examples
A
- Alpha-helix or Beta sheet
- Alpha-Helix proteins
- Myoglobin has almost all alpha helices
- Hydrogen bonds hold alpha helices together
- Beta sheet proteins
- Antibodies and Tcell receptors largely from beta sheets
- Most proteins have both
4
Q
Tertiary Structure
- What it is
- 5 examples
A
- 3D shape of polypeptide chain
- Polar/Nonpolar Interactions
- Hydrophobic aa point inward
- Hydrophillic aa point outward
- One of most important features in determining tertiary structure in proteins
- Hydrogen Bonds
- Van der Waals Forces
- Significant when many atoms can line up closely
- Most important in nonpolar amino acids
- Ionic Interactions
- Certain amino acid in ionic form
- Example: glutamate
- Disulfide Bonds
- Secreted proteins only
- Inside cell=reducing environment/disturb bond
5
Q
Quaternary Structure
A
- Contain 2 or more polypeptide chains
- Each polypeptide chain=subunit
- Example: hemoglobin, 4 subunits
- Example: collagen, 3 subunits
6
Q
Prosthetic Groups
A
- nonprotein group forming part of or combined with a protein
- Example: myoglobin and prosthetic group heme
7
Q
Why do hydrogen bonds form so frequently in polypeptide chains?
A
- Oxygen and nitrogen in peptide bond
- Polar region in peptide bond
- Hydrogen bonds form due to the mutual of attraction of two such oppositely charged regions
8
Q
Specific Binding
- Importance
- Specificity based on… (4 things)
A
- Function of almost all proteins is based on their specific binding of other molecules
- Specificity based on several features
- Complementary shape of protein and ligand
- “Lock and Key”
- Polar/Nonpolar interactions
- Electrostatic attraction
- Hydrogen bonding
- Complementary shape of protein and ligand
9
Q
Allosteric Regulation
A
- Regulatory molecule bind site OTHER THAN catalytic site
- Cause 3D shape change

10
Q
Protein Kinase
- Picture
- What works by activating protein kinases?
A
- Transfers phosphate from ATP to protein
- Hormones and most growth factors work by activating a protein kinase

11
Q
Protein Phosphatase
A
- Removes phosphate group
- Return protein to original state
12
Q
GTP Binding Proteins
- Picture
- Active vs. Inactive States
A
- Important in the action of many hormones and other physiological regulatory molecules
- Active=GTP, Inactive=GDP
- Reactivated by GTP replacing GDP on the molecule

13
Q
Protein Secretion Sequence
- Picture
A
- Proteins synthesized at ribosome
- For use in cell, ribosome in cytosol
- Secreted: 1st amino acids in polypeptide=signal sequence
- Signal sequence made
- Synthesis stops
- Ribosome dock at rough endoplasmic reticulum
-
Protein threaded into RER
- Signal sequence cleaved
- Enzymes cut proteins elsewhere, most secreted proteins modified
-
Vesicles with protein bud from RER
- move to the Golgi apparatus
- Fuse to first pita stack
- Vesicles bud off and add to other pita stacks
- At other end of Golgi, secretion vesicles bud off
- Move to plasma membrane
- Attach and release via exocytosis
- Often need signal for release

14
Q
Secretion of Collagen
- Picture
A
Made in fibroblast
- 3 tight packed polypeptide chains, linear and stiff
- 3 chains synthesized separately
- Wind around each other in RER
- Procollagen with tangled ends
- Relatively soluable
- If aggregate in cell, fatal
- Secreted from secretion vesicles into a groove at the surface of the cell
-
Procollagen proteinases cleave tangles
- Forms tropocollagen
- Less soluable and straight
- Start adhering to each other within the groove
- Form fibrils
- Spun out of the groove
- Crosslinked into strong fibers

15
Q
Glycoproteins
A
- Proteins with carbohydrates added
- Called glycosylation
- Some in RER, most in Golgi
- Makes polar, stable, soluable (Ex: Growth hormone)
- Also hold more water (Ex: mucus)
16
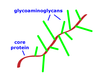
Q
Proteoglycans
- Picture
- Structure/Part
- Two places found
- Properties
A
- Carbohydrate glycoaminoglycans (GAGs) added
- Monosaccharides are linear
- GAGs along protein core to form “bristle brush”
- GAGs highly negative charge
- Often aggregate into huge complexes by adding to long linear molecule (Ex: hyaluronan)
- Take up lots of water, make gel like
- In the interstitial fluid
-
Cartilage has proteoglycans, strength from collagen
- Proteoglycans make it shock absorbing and springy (Ex: intravertebral discs)
17
Q
How do newly synthesized membrane proteins wind up embedded in the plasma membrane?
A
- The new membrane proteins thread into the RER membrane as they are being synthesized
- The membrane of the endoplasmic reticulum moves through the Golgi apparatus and becomes a secretion vesicle
- Secretion vesicle fuses with the plasma membrane, the newly synthesized membrane protein is added to the plasma membrane.
18
Q
Membrane Proteins
- Endocytosis
- Endosomes
- Picture
A
- removed from the plasma membrane and then returned in a relatively short time
- Removed through endocytosis
- Fuse with a larger, membranous structure called an endosome
- Budding from endosome, vesicles can bind to plasma membrane
- Endosome=quick means of forming new secretion vesicles

19
Q
Filling Orbital Shells
A
- first shell closest to the nucleus has only one orbital
- 2 e-
- next two shells have four orbitals
20
Q
Amphipathic
A
Both a polar region and a nonpolar region coexisting in the same molecule
21
Q
Why We Breathe Oxygen
A
- Serves as a repository for all those electrons that have lost energy in the process of generating ATP
- Electron from C-H to O-H in water, less energy
22
Q
First Law of Energetics
A
- energy is conserved
- energy transformed, but total amount of enery stays the same
- Quantity
23
Q
Second Law of Energetics
A
- Quality
- Order→Disorder
- entropy of the system increases with time
- less ordered system with time
- Reactions can occur if there is an increase in entropy
- Mostly heat, some more molecules
- capable of powering work or synthesis
- Negative free energy change
- -delta G
- “spontaneous”
- coupled reactions if overall negative free energy change
- factor causing a negative free energy change is almost always a net release of heat
- Glucose+oxygen=more molecules and heat

24
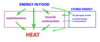
Q
Flow of Energy Through Body
A
- As it flows through the body, chemical synthesis and work are possible.
- Energy leaves the body in the disordered form of heat at body temperature
- May or may not be a net change in the amount of energy stored within your body
25
Rate of Energy Expenditure
* Definition
rate at which carbohydrates, fats and proteins are broken down.
26
Direct Measurement of Energy Expenditure
* proper energy unit
* Energy measured by heat
* Rate at which heat is formed
* kcal/min
* amount of heat energy required to heat one kilogram of water one degree Celsius
* equal to a dietary calorie
* proper energy unit kjoule
* equal to 0.24 kilocalories
* Theoretically correct method of measurement
* Not practical
27
Indirect Measurement of Energy Expenditure
* Measure how fast body uses oxygen
* one glucose to carbon dioxide and water, exactly six oxygen used
* 4.8 kcal/l O2
* typical mix of carbohydrates, fats and proteins
28
MET
* metabolic equivalent (MET)
* 1.0 kcal/hr per kg
29
VO2 Max
* Over 15 min, more work required until go no further
* Measure O2 at that point
* mL O2/min\*kg
* Brief periods: ATP can be generated without use of oxygen in mitochondria
* Longer periods: rate at which ATP is generated is directly proportional to the use of oxygen
* depends on size, genetics and level of training for endurance exercise
* older patients found that VO2 max is one of the best predictors of mortality
30
Respiratory Quotient (RQ)
* Equation
* Only carb, only fat, mixture
* Double labeled water technique
* RQ = carbon dioxide released/oxygen consumption
* Only carb, RQ=1
* Fats, RQ=0.7
* RQ=0.8-0.9
* Doubly labeled water technique
* water with rare isotopes of both oxygen and hydrogen
* rate at which both isotopes disappear from the body is followed
* Loss of H: loss of water in urine
* Loss of O: rapid loss of CO2
* Ratio: allows a calculation of the rate at which carbon dioxide is being produced and exhaled from the body
31
Carbonic Anhydrase
* Responsible for rapid oxygen to carbon dioxide in labelled water experiment
* In RBC
* CO2 + H20 \<—\> H+ + HCO3-
* fastest enzyme in the body
32
Reactions With Glucose
* Picture
* Rate Limiting Factor
* O2 delivery to muscles
* Run glycolysis, make lactic acid
* Lactic threshold=anaerobic threshold
* Acidity of blood increases, respiratory things go on
* Oxidative Phosphorylation
* Make ATP, add e- to O2

33
Water Polar Covalent Bonds
* Polar molecules attract a layer of water around them
* C and H share e- really well
* O and N attract e- more (polar covalent bonds)
34
Water Solvent Properties
Like dissolved like
35
Water Hydrogen Bonding
* Adjacent water molecules attract one another
* In liquid, transient bonds
* High surface tension
* Important in respiratory physiology
36
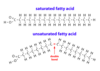
Fatty Acids: Saturated and Unsaturated
* nonpolar molecules
* oxygen and a hydroxyl group at one end
* Saturated
* All single bonds
* linear
* Unsaturated
* One or more double bond
* Bent, locked carbons, "kink"
* Classified by location of double bond from end of molecule *without* oxygens
* Ex: n-3 or omega-3
37
Cis/Trans Fatty Acids
* Double bond cannot rotate
* "kink"
* Cis=more pronounced bend
* Cis fatty acids not solidify as readily
* Cis isomers cannot line up next to one another
* Trans=naturally uncommon
* when polyunsaturated fatty acids from plants are "partially hydrogenated" chemically
* make solid and improve shelf life
* Increased risk of heart disease
38
n-6 and n-3 Fatty Acids
* electrical excitability
* polyunsaturated fatty acids
* Can serve competing functions
* Body doesn't make, so you eat them
* essential fatty acids
* Plant and plant oil
* alpha lineolic acid from soybean, canola, flaxseed and walnut oils
* n-6=linoleic acid
* make to arachidonic acid
* made into eicosinoids (regulatory)
* paracrine, act locally
* n-3=alpha linoleic acid
* EPA and DHA
* Fish oil
* paracrine
* Appropriate stimulus, the fatty acid is freed and then converted into a certain paracrine
* Directly affect opening and closing of ion channels and thus the electrical excitability of membranes
* cardiac arrhythmias
39
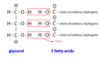
Triacylglycerols
* Picture
* three fatty acids joined together by one glycerol molecule
* adipose tissue, plant oils
* unsaturated fatty acids in triacylglycerol makes it more fluid
* Triacylglycerols are sometimes referred to as triglycerides
40
Phospholipids
* 2 ends to molecule
* polar/nonpolar
* amphipathic
* membranes
* see figure for parts
* "small polar"-ex: choline
* keep happy through micelle and bilayer
* break surface tension, ex: soap, in lungs

41
Cholesterol
* lipid molecule
* compact/rigid
* insoluable
* only 1 oxygen
* plasma membranes

42
Derivatives of an n-6 Polyunsaturated Fatty Acid
* Regulatory molecules from n-6 arachidonic acid
* 20 carbons
* Polyunsaturated
* eicosanoids
* defense against damage and pathogens
* inflammation and hemostasis
43
PLA2
* Stimuli activates membrane enzyme called phospholipase A2
* Ex: condition that causes, or threatens to cause, tissue damage
* acts on a membrane phospholipid
* Arachidonic acid now substrate
44
COX1, COX2
* cyclooxygenase
* produce a regulatory molecule that is either a prostaglandin or a thromboxane
* bend arachidonic acid into a hairpin
* All cells have different "further enzymes"
COX1
* normally present
* normal physiology
* inhibit stomach secretions (prostaglandins)
COX2
* Not normal physiology
* Threaten tissue damage
* Inflammation
* Induced
* TNF-alpha and IL-1 induce COX2
* Inhibited by glucocorticoid steroids
* COX2 specific inhibitor
* Cardiovascular repercussions
* Some removed, others used sparingly

45
LOX
* inhibitor
* lipoxygenase (LOX)
* regulatory molecules in the family of the leukotrienes
* Inflammatory
* Hay fever, asthma, ibuprofen not work
* LOX inhibitor
* Zileution
* monteleukast, prevent leukotrines from binding receptor
* eicosanoid regulatory molecules=paracrines
* degraded too rapidly

46
COX Inhibitors
* nonsteroidal anti-inflammatory drugs (NSAIDS)
* aspirin, ibuprofen, and naproxen
* celecoxib, selectively act on COX2
* rofecoxib removed because of cardiovascular repercussions
* Aspirin
* covalently modifies COX
* mall amounts of aspirin affect platelets for more than a day
* platelets moving through the intestines have their COX permanently blocked
* platelets lack a nucleus, new COX forms only with the synthesis of new platelets
* related to NSAIDs, acetaminophen
* supresses pain and fever
* little effect on inflammation and the secretion of stomach acid
47
Derivatives of n-3 Polyunsaturated Fatty Acids
* beginning with EPA and DHA
* incorporated into membrane phospholipids and then released into the membrane
* Released by PLA2
* Much less understood
* interfere with arachidonic acid binding w/ COX and LOX
* Resolve inflammation
* can affect the electrical excitability of cardiac muscle cells
48
How To Move Fat
* Break down TAG and release FA
* Very small amount soluable in blood
* FA bind to transporter to get into the tissue
* increase by exercise and cold exposure
49
Lipoprotein Structure
* Not molecules, they are a particle
* Solve lipid/water soluabilty by utilizing amphipathic nature of phospholipids
* single layer of phospholipid molecules on their outside, surrounding a central core
* outside of the phospholipid molecule is polar/water compatible
* nonpolar portion inside, compatible with nonpolar core of lipoprotein
* esterified cholesterol
* super nonpolar cholesterol
* Fatty acid on the oxygen
* normal cholesterol is found in the outer layer of phospholipid
* outer layer, protein molecule, apolipoprotein
* amphipathic, stabilizer
* Each lipoprotein identified through differing apolipoprotein

50
Lipoprotein Movement
* Some transport dietary lipids from the small intestine to adipocytes and the liver
* Some transport cholesterol between different part of the body
* At target cell:
* apolipoprotein binds to a receptor
* lipoprotein taken up by receptor mediated endocytosis
* Sometimes enzyme on capillary wall, lipoprotein lipase
* unloads triacylglycerol
* breaks TAG into FAs and glycerol
51
HDL and LDL
* LDL
* liver to cell
* HDL
* back to liver
* differentiated by apolipoprotein
52
Immune Cells Using Phagocytosis
* 4 of them
* Neutrophils
* abundant in the blood, quickly enter tissues, and phagocytize pathogens in acute inflammation.
* Macrophages
* related to monocytes in the blood. These longer-lived cells predominate in chronic inflammation. They also release some important inflammatory paracrines
* IFN-gamma increase macrophage production of superoxide, type 2 interferon
* Dendritic Cells
* elaboration of a specific immune response rather than for directly destroying the pathogens
* B Lymphocytes
* small amount of phagocytosis in these cells is often necessary in order for them to develop into cells that release antibodies
53
Phagocytosis: Sequence of Events
* lysosome derived from
* neutrophil or macrophage flowing around the pathogen and engulfing it
* enclosed in a phagosome
* fusion of lysosomes with the phagosome
* forms phagolysosome
* Lysosome derived from Golgi
54
The Dangers of Oxygen
* Electrons from food to NADH or NADPH
* Need oxygen to put electrons and form water
* Oxygen is electron garbage can
* Each step of electron transport chain hold electron just a little tighter
* Take a little energy at each step and form ATP
* One electron in an orbital, form a radical
* Small amounts made in metabolism
* Highly reducing and can cause tissue damage
* 21% of air, not full of water we would burn up
55
Destruction of Microbes: Oxygen Radicals
* Overview
* phagocyte oxidase in membrane of phagolysosome
* generate oxygen radicals
* single electrontaken from NADPH and added to oxygen
56
Destruction of Microbes: Nitric Oxide
* Nitric oxide synthase synthesizes nitric oxide
* reacts with superoxide to create further molecules that damage biological molecules
57
Destruction of Microbes: Anti-Microbial Proteins
* 2 Specific Examples
* Lysosomes contain several proteases
* broad spectrum enzyme elastase
* important or even essential for killing various bacteria
* lysozyme
* attack gram+ cell wall
58
Destruction of Microbes: Anti-Microbial Peptides
* Defensins attack bacterial membranes
*
59
Destruction of Microbes: Binding Proteins
* Lactoferrin binds iron
* Another binds vitamin B12
60
Destruction of Microbes: Hydrogen Ion Transport
* Transporters for hydrogen ions (a second role of the oxidase) acidify the phagolysosome
* important for the action of the proteases
61
Destruction of Microbes: Picture

62
Phagocyte Release of Regulatory Molecules
* small proteins, cytokines
* released by WBC, such as macrophage
* cytokines=paracrines
* certain cytokines act more systemically, ex: cause fever
* TNF-alpha and IL-1
* potent inflammatory cytokines
63
Phagocytosis Identification of Pathogen
* Neutrophils and macrophages ability to recognize microbes
* innate receptors bind foreign molecules
* act immediatly as microbe enter body
* **opsonin**, bind microbe and increase phagocytosis
* most important is antibodies
64
Listeria
* Escape phagosome into cytosol
* Cause food poisoining, can be fatal
* Very bad for fetus
* Whole cells must be destroyed to kill Listeria
65
Tuberculosis
* prevents lysosomes from binding phagosome
* if macrophage not activated, bacterium live inside the cell
* macrophage surround infected macrophage, creating a **granuloma**
66
Anthrax
* Forms a capsule
* Enter from lungs/cut in skin, to lymph node, begin dividing
* cause sepsis
* TNF-alpha and IL-1 released and cause hyperinflammation
* Form widespread clots, called disseminated intravasular coagulation
67
Flow Chart of Oxygen Radicals in Phagocytosis
* Superoxide radical moderately reactive
* Converted to hydrogen peroxide
* can cause damage
* myeloperoxidase in phagolysosome of neutrophil
* Hydroxyl radical damage macromolecules like DNA, proteins and lipids
* iron in blood bound to transferrin and stored in blood protein as ferritin
* neutrophil and macrophage are messy eaters
* catalase in cytosol protect from messy eaters

68
Nitric Oxide
* 3 forms
* also a radical and is highly reactive
* lasts only seconds in body
* made from the amino acid arginine by **nitric oxide synthase**
* neuronal, endothelial, inducible
* inducible form in the immunological form
* Lots of nitric oxide can cause loss of blood flow, shock
69
Protection From Oxygen Radical
* catalase, superoxide dismutase, etc.
* proteins that bind Fe++ and Ca++
* antioxidants
* ex: vitamin C
70
Chronic Granulomatous Disease
* NADPH oxidase in phagocytosis in defective
* Neutrophil and macrophage can't make free radical
* can't kill bacteria
* other macrophages glom around the infected macrophage
* see granuloma in chest xray of TB, can clog GI tract
* recurrent infections
* catalase making bacteria=granuloma, can really wreck havoc
* Treatments:
* antibiotics
* Bone marrow transplant
* IFN-gamma infusion
71
Hydroxyl Radical and Lipids
Creates a Chain reaction that can destroy a whole patch of membrane

72
Bone Marrow and Key Cells
* Colony Stimulating Factor
* Gives rise to cells engaged in the defense mechanisms
* babies=all bones
* adults=replaced by fat, only in central bones (ex: ischium)
* hematopoietic stem cells give rise to:
* lymphoid stem cells
* lymphocytes to recognize foreign things
* myeloid stem cells
* other blood cells like RBC and macrophage
* Colongy stimulating factor
* Stimulate stem cells
* GM-CSF (drug)
73
Lymphocytes
* 5 of them
* make specific molecules to identify foreign antigens
* antibodies and t cell receptors
* B-Lymphocytes:
* Produce antibodies
* Helper T Lymphocytes
* Have T-cell receptors
* activate macrophages
* help B cell develop their response
* Cytotoxic T Lymphocytes:
* Kill virus infected cell
* Kill cancer cell
* Regulatory T Lymphocytes:
* Suppress T cell
* Natural Killer Cell:
* kill virally infected cells
* part of innate response
74
Fluid Compartments in Body
* Intracellular Fluid:
* Inside cells
* for 70kg person, about 28 L
* Extracellular Fluid:
* Outside cells
* about 14 L
* Divided into two compartments
* Blood Plasma:
* Fluid part of blood
* About 3 L
* Interstitial Fluid:
* outside blood vessels and bathing cells
* lots of nutrients
* about 11 L
* Capillary=one layer of endothelium cells
* permeable to molecules smaller than proteins
* so plasma-blood protein and blood cells=interstitial fluid
75
Lymph
* Derived from interstitial fluid
* lymphatic capillary
* much more permeable than blood capillaries
* thin walled
* flap valve so even microbe go in lymph
* lymphatic vessels drain away excess interstitial fluid
* about 2 L/day
* thin walled w/ small amount of smooth muscle
* flow created by muscle contraction and lymph formation
76
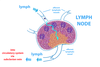
Lymph Nodes
* Enter from periphery into subcapsular space
* Move towards the concave side
* microbe and dendritic cell can also enter lymph node
* lymph encounters lymphocyte to cause specific immune response
* B cell response: lymphoid follicles
* T cell response: paracortical areas
* high endothelial venules: thin-walled vessels in paracortical area after the capillaries
* Cube cell rather than pancake cells
* lympocyte bind cell adhesion molecules, cross endothelium, enter lymph node
* Lymphocytes move between lymph nodes
* Lymphocyte encountering infection recruite more lymphocytes by expressing more cell adhesion molecules
77
Spleen
* White pulp
* same types of cells as lymph node
* focus on microbe in blood
* not typical
* around the thinnest arteries
* Red pulp
* destroys RBC
* macrophage that engulf RBC
* healthy RBC can get in venous sinus
* old RBC can't get in, eaten, got to get through lattice work and not flexible
78
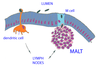
MALT
* Picture
* Deal with microbe in lumen of GI tract (could be others)
* More suseptible because lined with living cells
* Mucosal immunity
* antibody into lumen
* Cluster of cells in interstital fluid directly below epithelia
* Some large
* Tonsils and Peyer's patches
* have follicles
* Cells move between MALT and lymph nodes
* Microbes rather bind mucosal surface and chill there than enter the epithelial interstital fluid
* Cover with mucus
* Dendritic cell extensions capture microbe in the lumen
* Epithelial M cells engulf a microbe and transfer its molecules to the MALT
* Lymphocytes embedded in epithelium lining the intestines
* Respond to more commonly encountered microbes
* Make IgA
* Dimer can bind basal surface and be transferred to apical surface
* Prevent microbe binding, agglutinatation
79
Cutaneous Lymphoid Tissue
* epidermis embeded with Langerhans cells
* type of dendritic cells
* catch microbe and travel to the lymph node to start adaptive response
* under epidermis also macrophage and tcells (like there is elsewhere in the body)
80
Thymus
* organ lying just above the heart
* tcell development and selection
81
Megakaryocytes
* bud point for platelets
* in bone marrow
82
Platelet Structure
* perfect disc
* if not in cardiovascular, cause activation
* Secretion Vessicles Inside:
* Dense Granules: Contain ADP
* Alpha Granules: various proteins including thrombin, glycoprotein IIB/IIIA, VWF, growth factors
83
Platelet Activation
* Absence of activating factors and release of prostacyclin (prostaglandin I2) by healthy endothelium supports this state
* Break in endothelium triggers activation
* collagen, thromboxane A2, ADP and thrombin
* Main things that happen as a platelet is activated:
* Exocytosis of the dense granules and alpha granules.
* Activation of the membrane enzyme phospholipase A2. This leads to the formation of thromboxane A2 (TXA2)
* Change in shape to a more amorphous form with projecting fingers.
* Platelets adhere to one another and to collagen, forming a platelet plug. On the surface of the activated platelet are VWF receptors and glycoprotein IIb/IIIa. The latter is a receptor which binds fibrinogen. The VWF is like a glue sticking platelets to collagen. The binding of fibrinogen by glycoprotein IIb/IIa causes platelets to adhere to each other. The fibrinogen that connects two platelets via these receptors is found in the blood and also a little is released from the alpha granules.
* Coagulation reactions are promoted at the surface

84
Name three factors that not only are produced or released as a result of platelet activation but also cause platelet activation.
ADP, TXA2, Thrombin
85
Some people take one "baby" aspirin a day to reduce the risk of a myocardial infarction. What molecules are affected by the aspirin?
COX1 is inhibited in platelets, leads to less TXA2, less platelet activation,
86
Blood Coagulation Reactions
* Coagulation and platelet activation form a clot
* Set in motion by damage to a blood vessel
* Contact blood with tissue factor
* tissue factor everywhere with cancer metasis
* clots and use up platelets
* Found especially on cells in the tissue surrounding blood vessels
* Each step causes "factor" to be activated into proteolytic enzyme
* In turn, promotes the next steps
* Prothrombin--\>Thrombin
* Fibrinogen--\>Fibrin
* Fibrinogen=soluable and dispersed
* Fibrin associate into fibrils
* fibrous part of clot
* Fibrinogen:
1. Fibrous part of clot
2. "platelet glue"
* platelet plug okay for small breaks, coagulation for larger
* fibrils trap activated platelets
* Release thrombin, enhancing platelet activation

87
Prevention of Clotting
* 6 ways
* Intact endothelium
* VWF, collagen and tissue factor stay away from platelet
* Prostacyclin (PGI2)
* made when endothelium intact
* acts on platelets (TXA2 is the opposite)
* NO keeps vessels dialated
* Tissue factor pathway inhibitor
* Released by endothelium
* Thrombomodulin
* contain factor I (destroy them)
* On endothelium
* binds thrombin and protein C
* protein C activated and inactivates clotting factors in blood
* Heparan proteoglycan (heparin)
* On endothelium
* binds and activates anti-thrombin
* inactivates thrombin

88
Platelet Plug Starts to Form
* Picture
* Break in endothelium causes platelets to contact collagen
* platelets activated
* Platelets adhere to one another and subendothelial tissue
* via glycoprotein IIb/IIIa and VWF receptors
* Platelet plug may be adequet
* Platelet release ADP and activated by ADP

89
Coagulation Reactions Begin at Surface of Platelet
* Picture
* Coagulation reaction start occuring rapidly
* Tissue Factor is exposed
* Surface of activated platelets provides the environment for cascade leading to prothrombin--\>thrombin
* Clot consists of interlaced fibrin fibrils and activated platelets

90
Hypercoagulability
* 3 examples
* Injury to the endothelium, in general, tends to activate platelets
* Might be inappropriate activation
* Ex: developing atherosclerotic plaque in an artery
* Ex: turbulent blood flow
* Ex: damage to endothelium from an immunological cause
91
Slow Flow and Clots
* 3 Examples
* accumulation of activated clotting factors
* inhibitors no longer correctly inhibit
* Ex: atrial fibrillation
* Not contract rhythmically
* Atria stretched with areas of poor flow
* Left atria clot that can travel to brain
* Ex: Stasis blood flow in legs
* venous thromboembolism
* Ex: disseminated intravascular coagulation
* May arise from sepsis, cancer, trauma
* small clots form, deplete platelets and clotting factors
* hemorrhaging can result
92
Coagulation Deficits
* 5 Examples
* Thrombocytopenia:
* low platelets
* capillaries easily bruise
* purpura=blood leaking out of capillaries
* petechiae: small, round, red to purple spots
* ecchymoses: later than 3 mm spots
* Genetic Lack of Certain Clotting Factors:
* hemophilia
* Lack of VWF
* Impaired synthesis of clotting factors
* Vitamin K deficiency
* Ex: Newborns
93
Anti-Platelet Activation Drugs: Aspirin
* Covalent affect
* Permanently blocks COX enzymes
* blocks TXA2 synthesis
* Once platelet lose COX--\>done
* no nucleus
94
Anti-Platelet Activation Drug: Clopidogrel (Plavix)
* blocks ADP receptor
* reduce clots in blood vessels damaged by atherosclerosis
* used in patients at risk of a myocardial infarction
*
95
Anti-Platelet Activation Drugs: Dipyridamole
* Increases level of adenosine by inhibiting uptake by platelets
* Adenosine blocks affect of ADP
* Incidentally, more ADP in blood leads to more adenosine, which in turn inhibits ADP
96
Anti-Platelet Activation Drugs: Abciximab (Reopro)
* enzyme that block glycoprotein IIb/IIIa receptor
* on outside of activated platelet that bind fibrinogen
* so decreases stickiness of platelets
* needs to be injected, not used for everyday, used in invasive surgery
* monoclonal antibody
97
Anti-Coagulation Drugs: Warfarin (Coumadin)
* Prescribed for atrial fibrillation, stagnant blood/clots
* Vitamin K antagonist
* Needed for thrombin synthesis (prothrombin activity)
* Thrombin not formed as quickly
* Kills cows by hemorrhaging (eat the sweet clover)
* Have to measure the prothrombin time monthly, need to twek all the time
98
Anti-Coagulation Drugs: Direct Inhibitors of Thrombin and Factor Xa
* Thrombin inhibitors
* Dabigatran (Pradaxa)
* Factor Xa blocker
* Apixaban (Eliquis)
* no constant tweaking
* block the active substances directly
* direct inhibitors continue working even if the factors are increased
* (not be quickly reversed)
*
99
Anti-Coagulation Drugs: Heparin
* Same function as endothelial heparan proteoglycan
* Leads to anti-thrombin being formed
* Chain of highly charged carbohydrates
* Low MW (enoxaparin, etc)=more expensive=more purified
* more refined product with only the smaller sized molecules provides a more measured effect
* since it has a bigger effect on a blood coagulation factor than on prothrombin itself
* It is released from mast cells
* Injected, so used when hen a short term, rapid effect is desired
100
Drugs That Break Down Clots
* following a myocardial infarction or a stroke
* Careful with strocks, because some are hemorrhagic
* **tissue plasminogen activator (tPA)**
* normally converts plasminogen to plasmin in the blood
* Plasmin is an enzyme that breaks down fibrin
* normal enzyme that starts the destruction of a clot after it forms
* injected drug
* with 3 hours
* maybe later if directly with catheter
* Recombinant DNA technology to manufactor
* **streptokinase**
* ****enzyme purified from bacteria
101
Recognition Molecules on Phagocytes
* Ex: Toll-like recetors
* Bind molecules on microbes
* 11 different molecules in this category
* activates various genes important for orchestrating a innate immune response
* Ex: Mannose receptors
* recognize repetitiveness on cell wall of microbe
* 3 or 4 more known types of known recognition molecules on macrophages
* Normally expressed
102
Soluable Recognition Molecules
found in the blood plasma
* Opsonins
* Ex: C-reactive protein
* liver makes these, put in blood during infection,
* bind microbe, like badge saying eat me to macrophage
* C-reactive protein shows amount of inflammation
* Ex: Mannose Binding Lectin
* C3b
* Antibodies are best opsonins
* Macrophage have molecules that bind CRP and MBL
103
Acute Phase Proteins
* Macrophage--\>TNF-alpha--\>liver--\>C-reactive
* Cytokines start fever (IL-1, TNF-alpha)
* Not cook bacteria
* Speed up immunological responses
* Not easy to pinpoint why fever occur
* Macrophage also release chemokines
104
Complement System
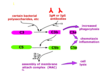
* blends and makes better
* cascade of events at the surface of the pathogen
* proteolysis
* innate (bacterial polysaccharides) and specific (antibodies)
* Fc of antibody allows C1 to bind (1st complement protein)
* C3b (protein) stick to microbe surface
* opsonin
* C3a small peptide
* Chemotatic factor and inflammatory paracrine
* Multiple C9=elongated, core protein in cell wall
* Thus complement system trigger a consetellation of events
* opsonization
* chemotaxis
* inflammation
* lysis, apoptosis
105
Natural Killer Cells
* virus--\>hijacked cell wall
* macrophage and neutrophil outside relm of expertise
* NKC help cells commit apoptosis
* apoptosis=membrane intact--\>macrophage eats it up
* necrosis=cell die and break apart
* NKC bind to cell w/ virus (unkown how recognized)
* release signal to create apoptosis
* has to be cautious
* won't get rid of serious infections
* slows things down
106
Epithelial Surfaces: Mucous Membranes, Antimicrobial Peptides and Proteins
* Mast Cells
* release histamine
* Antimicrobial proteins/peptides (in lysosome as well)
* lysozyme (etc.)
* defensin
107
Eosinophils
* made for helminths/worms
* multicellular parasites
* need something special against these
* identify, release highly basic proteins--\>attack helminth
* acute inflammation
* increase endothelial permeability
* when neutrophils or IgE response don't work
108
Initial Encounters With Pathogen
* two types of phagocytic cells normally present in interstitial spaces
* dendritic cells and the resident macrophages
* always out in the tissues waiting to encounter any microbes
* Dendritic Cells
* collecting antigen
* carrying it to lymphocytes in lymph nodes, spleen or MALT
* Pull in projections
* serve as antigen presenting cells
* peptides from the phagocytized protein put on the surface of the cell attached to MHC II molecule
* "present" the antigen to helper T cell
* Macrophages
* processes begin within minutes
* destroy pathogens
* especially long term infections that turn into chronic problems
* resident; scattered through blood
* start phagocytizing when in interstitial fluid
109
What, in general, is a cytokine?
* Protein regulatory molecule that helps coordinate an immune response
* Paracrines
* Released by lymphocyte, macrophage or other WBC
110
Roles of Cell Adhesion Molecules
* Need recruits-the neutrophils
* lots in the blood
* short lived (hrs to days)
* First bind endothelium of blood vessels
1. TNF-alpha and IL-1 from macrophages cause endothelial cells to express two types of cell adhesion molecules
2. selectins lightly tether the neutrophil to the endothelium. Slow their roll
3. tighter binding occurs. ICAMs
4. ICAMs bind tightly to molecules known as integrins on the neutrophils
111
Diapedesis
* Increased permeability by cytokines
* Squeeze between adjacent endothelial cells
* Fluid leakage (even blood proteins), accumulate in tissues (edema)
* increases lymph flow

112
Chemotaxis
* Seek out the microbes
* Follows signals, sniff it out
* chemotactic factors
* chemokines (NOT cytokines)
* promotes chemotaxis of phagocytes and lymphocytes
* small proteins
* certain bacterial molecules
* C3a and C5a peptides
* help neutrophil and macrophage smell infection out
* Chemokine released by macrophages and endothelial cells

113
Monocytes
* With time, come to infection site
* Once in tissues, called macrophages
* important for chronic infections
114
Macrophages: TNF-alpha, IL-1, chemokines
* inflammatory paracrines
* powerful, widespread effects
* Coordinated innate response
115
Virally infected cells: INF-alpha, INF-beta
* Type 1 interferons
* released by virally infected cells
* also by concerned lymphocytes
* induce state that make harder for virus to infect cell
* slow down viral replication
* increase MHC1 molecules on cell surface
* cut down on cell division, tell body to be cautious
* INF-alpha used as drug for Hep B
* INF-beta used as drug for MS
116
Many Location of Cytokines and Other Regulatory Molecules of Innate Immune System
* Paracrines
* Mast cells
* throughout body, under epithelia
* large vessicles with histamine and other inflammatory paracrines
* also release PG D2, several LTs and TNF-alpha
* Factors associated with tissue damage trigger the exocytosis
* sometimes specific immune response
* Eicosanoids
* various arachidonic acid derivatives
* prostaglandins (notably PG D2) and leukotrienes (LT) can be important, depending on the tissue
* aspirin and NSAIDs
* C3a and C5a
* nitric oxide, certain platelet products, kinins, and certain other substances we will not discuss (serotonin, etc)
* bradykinin as vasodialation
117
Events in Acute Inflammation
* time frame
* what 3 things happen
* seconds, minutes, hours, and days
1. Increased blood flow due to dilation of blood vessels (arterioles) supplying the region
2. Increased permeability of the capillaries, allowing fluid and blood proteins to move into the interstitial spaces
3. Migration of neutrophils (and perhaps a few macrophages) out of the capillaries and venules and into interstitial spaces
118
Decribe What See and Why

* layer of neutrophils adhering to the inner surface of the blood vessel
* neutrophil=several lobed nucleus
* also observe neutrophils outside as well as inside the blood vessel
119
Describe What See and Why

* light micrograph of pus
* pus is packed with neutrophils
120
Chronic Inflammation
* primary cells of chronic inflammation are macrophages and lymphocytes
* still have increased blood flow and increased capillary permeability
* indigestible material may remain inside macrophages in vesicles for long periods
* important secretory cells releasing inflammatory paracrines, growth factors, and a variety of other proteins
* T Cells enter inflammed tissue
* activate macrophages
* important for difficult pathogens
* arise most frequently in the context of autoimmune diseases
* B Cells in inflammed tissue
* Make antibodies
121
Describe What See and Why

* macrophages distended with lipid from broken down myelin at the site of necrotic tissue due to a blocked blood vessel in the brain
* large amount of nearly clear cytoplasm
* continue to engulf more, ven if they can't digest all the material phagocytized
122
Describe What See and Why
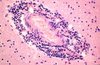
* brain tissue in multiple sclerosis
* lymphocytes emerging from the venule
123
Describe What See and Why

* thyroiditis in Hashimotos disease
* chronic inflammation destroys the thyroid gland
* Note the lymphocytes
124
Granulomatous Inflammation
* macrophages collect in layers
* macrophages will fuse, forming giant cells
* structure called "granuloma"
* characteristic feature of tuberculosis
* bacteria somehow prevent lysosomes from fusing with the phagocytic vesicles

125
Antigen
* abbreviated Ag
* proteins, peptide
* sometimes polysaccharide, DNA
* penicillin=haptan
* bind protein before becoming an antigen
* epitopes=antigen regions recognized by the immune system
* Specific binding to Ab or T Cell Receptor
126
Classes of Immunoglobins
* IgG (blood)
* IgM (blood)
* IgA (mucosal immunity)
* IgE (allergy, helminth)
127
Structure of Antibodies
* four polypeptide chains
* two identical light chains
* 2 domains
* two identical heavy chains
* 4 domains
* two identical binding sites for a specific antigen

128
Antibodies: Variable Domain, Constant Domain, Hypervariable Regions
* Variable Domain:
* Only 1 domain of each polypeptide that bind antigen
* 4 per antibody
* Bind different antigen
* Constant Domain
* Same within each antibody class
* Hypervariable Region
* Loops within the variable regions
* Change in amino acid sequence w/i this region
* Domains made of beta sheets
* don't mess with beta sheets or else lose 3D structure

129
Membrane Bound Antibodies
* Membrane bound IgG
* extra domains on heavy chain
* nonpolar, embed in membrane
* Opsonin
* Activate complement system as well
* IgE
* Constant domain stick to Fc receptor of eosinophil or mast cell
* Variable domain stick to helminth worm
* IgA
* Dimer held by J chain
* Lumen of airways
* epithelium take up into lumen
* stuck to bacterium/virus
* prevents bad guys from sticking/entering tissues
* swept away by cilia
* IgM
* Pentamer held by a J chain
* Aggluination
* Easier to pick up
* Heavy chain sticks up as an identifier
130
Heavy Chain Switching
* Make one type of immunoglobin
* Usually IgM
* Make variable domain
* specialize by change constant domain of heavy chain
131
B-Cell Maturation in Bone Marrow
* DNA rearrangement
* make own variable domains
* ten million different B cells from not much DNA
* 3 hypervariable regions
* each region has numerous possibilities
* Loop and chop extra DNA
* Some bind to molecule in body
* Selection
* Goes under apoptosis, "self-tolerance"
132
Chronic Granulomatous Disease
* Rare genetic disorder
* Inability of phagocytes to make hydrogen peroxide and other oxygen radicals
* Suseptibility to bacterial/fungal infections
* defective NADPH oxidase
* Treatment:
* IFN-gamma
* macrophage-activating-cytokine
* Expiremental: gene therapy
* Present approach: Remove neutrophil precursor cells from bone marrow, insert gene, infuse cells back.
133
Hemochromatosis
* Common genetic disorder of iron metabolism
* 0.5% American population
* 1 wrong aa in membrane protein, affects binding of transferrin to its receptor on cell, more iron is take up in the small intestine and stored in cells
* Transferrin and ferritin saturated
* excess iron react with oxgen radical to form hydroxyl radicals
* Symptoms:
* fatigue, diabetes mellitus, liver cirrhosis
* Diagnosis made late or never
* Treatment:
* Phlebotomy
134
Leukemia
* neoplastic (new, abnormal growth) disorder of bone marrow
* distorted production of white blood cells
135
Lymphoma
* Neoplastic disorder in lymph nodes of other parts of lymphatic system
* Ex: Hodgkin's disease
* Non-Hodgkin's disease
* Set of disorder, more common
* Symptom: adenopathy (swelling of "glands")
* Fever, night sweat and weight loss
* Malignancy can spread
* Unknown cause, viral etiology possible
136
Bone Marrow Transplant
* Donor found with close matching MHC alleles to recipient
* Bone marrow of recipient destroyed by chemotherapy/whole body irradiation
* Donor under general anesthesi and bone marrow aspirated from iliac crest
* filtered to remove bone spicules and other particulates
* Infused into circulatory system of recipient
* Stem cells find their way to bone marrow spaces and begin dividing and regenerating recipient's bone marrow
* Stem cells also from umbilical cord, although quantity might only be enough for a child
137
Autologous Transplant
* Donor and recipient are the same person
* Bone marrow obtained prior to treatment such as chemotherapy and then infused afterwards
138
Stem Cells from Peripheral Blood
* Give donor colony stimulating factor
* Ex: granulocyte macrophage colony stimulating factor
* Increase division of stem cells
* Enough stem cells, diplaced from bone marrow, show up sufficiently in blood to be collected
139
Graft Versus Host Disease
* Opposite of when a transplanted organ is rejected
* lympocytes from bone marrow transplant reject other tissue of recipient
* numerous body parts affected
* prevented by immunosuppressant drugs
140
Thrombocytopenia
* lower than normal level of platelets
* decreased production due to disease of bone marrow
* increase destruction by immunological or drug-induced causes, viral infections
* **Iatrogenic**: adverse condition as a result of a physician directed treatment
* Small points of bleeding in legs and mucosal membranes
* **petechiae**: small, round, red/purple spots
* **ecchymoses**: bigger than 3 mm
* **purpura**: hemorrhage into the skin with color change red-purple-yellow
141
Thrombosis
* Formation inside the lumen of the circulatory system of a thrombus
* mass of coagulated blood comprised of platelets and fibrin
* Attached to wall of the vessel
* (**Hematoma**=outside blood vessel)
142
Necrosis
* Caused by lack of blood flow to an area
* Area is called **infarct**
* Heart attack and strokes
* when thrombus in an artery, usually from atherosclerosis, breaks loose
143
Thrombus in Vein
* Usually deep veins in legs
* promoted by endothelial injury, stasis of blood in vessel
* prolong bed rest, heart failure, etc
* hypercoagulatibility due to cancer, etc.
* Thrombus break loose and begins moving through the blood, called **thromboembolus**
* ****Can travel to lungs, lodge, large venous thromboembolus can be immediately fatal
144
Tuberculosis
* Rising due to resistance
* higher risk in malnourished, crowded population, AIDS
* bacteria prevent lysosomes from fusing the phagocytic vessicles,
* bacteria multiply and then released
* T Cells relase cytokines
* IFN-gamma
* activate macrophage
* Crowd around bacteria, forming granuloma
* significant death of cells and fibrosis
* Strong immune response
* granuloma successful, necrosis=cheesy inside of granuloma
* Weak immune response (newborn)
* granuloma poorly formed and infection spreads
* Secondary TB
* previously contained in granuloma, but escape
145
Candida Albicans
* One of the most common fungal pathogens
* problem if impaired immune system
* normal flora disruption
* skin cleft and fold (dem fat rolls)
* **Oral thrush**
* ****creamy white growths on the mucus membrane of mouth
146
Hepatitis A
* RNA virus
* via feces
* liver--\> bile --\> feces
* Shellfish exposed to sewage
* replicate in liver...tissue damage
* hepatitis is acute, never develop into chronic disorder
* most people don't know had it
* immunity is lifelong
* effective vaccine
147
Hepatitis B
* Chronic disorder
* Most not aware of symptoms
* DNA virus, travel in blood, saliva and semen
* Can be mother--\>newborn
* Effective vaccine
* interferon-alpha can be used to stop infection if chronic hepatitis B develops
* Activating mechanisms allow cytotoxic T cells to destroy the infected liver cells
148
Hepatitis C
* RNA virus
* Clinical feature similar to Hep B
* more likely to become chronic
* viral infection slowly causing liver damage and cirrhosis


