Lecture 2 - Current developments in DFT Flashcards
(32 cards)
- EXC is … and needs to be …
- EXC is unknown and needs to be approximated
- What is the basic assumption of Local-density approximations (LDA)?
- EXC only depends on the value of the local electron density at one point
- How does an ensemble generalized LDA approximate XC energy as a functional of electron density?
- Exactly calculates ϵXCHEG energy density for a homogenous electron gas (HEG)
- What is a homogeneous electron gas?
- Box of homogeneous positive charge background filled with electrons (no atoms)
- These electrons spread out homogeneously allowing exact calculation depending on the density at that separation.

- Discuss the structural, elastic and vibrational properties as a result of the use of LDAs
- what approximation yields these results
- Generally, give satisfactory results
- Crystal bulk lattice (distance of unit cell vector) more accurate as usually underestimated
- Bulk moduli (E to compress/expand solid) too large but 10% error not uncommon
- Vibrational frequencies too high/stiff
- These are due to local approximation which compacts the entire system.
- Discuss some other problems with LDA’s
- Binding energies are too negative i.e.overbinding
- Activation energies are unreliable
- Band gaps, ionization energies and electron affinities are strongly underestimated.
- What are Generalized-gradient approximation (GGA’s) and how do they improve upon LDAs
- Similar form but a local gradient of electron density is included as well as the value of density
- This is to gain information on the local variation in neighbouring electron density at other positions
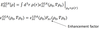
- What strategies can be taken in designing functionals
- Non-empirical functionals: satisfy constraints via certain known mathematical/physical boundary conditions (less accurate)
- Empirical functionals: satisfy a property by empirically fitting to yield prediction of a molecule/material property (more accurate – regularly used)
- What is the disadvantage of empirical design of functionals?
- Are derived from QM with good accuracy, however, are not truly ab initio.
- How does the bulk lattice constants and cohesive energies with GGA compare with LDA?
- Bulk lattice constants (unit cell vecotr between atoms): GGA increase due to more repulsive core-valence XC
- Cohesive energies (E released by binding atoms in to a solid): GGA reduction mostly due to valence effect, giving better description.
- How do Energy barriers of GGAs compare to LDAs
- Free energy of molecule better described with GGAs, reducing the degree to which the barrier is underestimated
- LDAs underestimate to a large extent.
Name an improvement GGAs make on LDAs
- GGA correct LDA overbinding, with less stiffness of tightly packed system
- Where do GGAs still fall short
- Still no long-range description of vdW forces as local approximation (same as LDA)
- As GGA favours low coordination (large gradient), can now interpret different E sites on a surface (LDA could not distinguish). However can do so incorrectly.
- For their simplicity, LDA/GGAs perform well for a large range of materials, marking ‘semi-…’ DFT as an important improvement in how we describe the …
- However major failures in the … of chemical reduction barriers and … … as well as the overestimation of … mark a need for further improvement.
- For their simplicity, LDA/GGAs perform well for a large range of materials, marking ‘semi-local’ DFT as an important improvement in how we describe the EXC
- However major failures in the underestimation of chemical reduction barriers and band gaps as well as the overestimation of polarizabilities mark a need for further improvement.
- What is an example of a GGA functional?
- Perdew-Burke-Ernzerhof (PBE)
- What is the general idea of meta-GGAs?
- Expand the local function dependence to occupied KS orbitals.
- 2nd derivative of KE added via T[ρ] of non-interacting electrons i.e. the product of the derivative of KS ψ of single particle space
- This leads to the function becoming explicitly orbital dependent.
- Still technically local derivative however accounts for many variations in different orbitals.
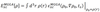
- How do meta-GGAs compare to LDA/GGA
- Better molecular binding E’s
- Better cohesive/structural description
- Band gaps still underestimated
- Long range vdW still not accounted for as still a local functional
(IMP) Describe the band-gap problem in GGA’s
- Underestimation of difference in ionisation energy/electron affinity due to self-interaction error
- Contrary to HF, VX and VH + VC don’t cancel for an H-atom
- Missing VX causes half electrons to move apart from each other instead of contracting in to a positive and negative ion in dissociation.
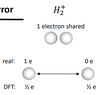
(IMP) What is the over localisation error also associated with the band gap problem?
- Electronic states are too localised as electrons do not sufficiently repel each other (Pauli repulsion missing)
- Electrons do not come close in contact unless they must (run away from themselves as orbitals more diffuse.
(IMP) Describe the problem of missing long-range correlation in local functionals
- Electron correlation VC treated incorrectly (due to local approximation)
- Leading to no long range interaction between non overlapping densities
- As a result, LDA/GGA/meta-GGA do not capture dispersion/vdW effects
(IMP) How does the error in delocalization affect how the ionisation energy and electron affinity are related to the energies of the KS states at this stage? Use a sketch to support your answer
- Energies of the KS auxiliary states do not equal the electron affinity and ionisation energy of the system as they should
- This is due to the presence of half electrons in local description causing a bowing of the curve instead of clear definition
- means half an electron in LUMO and HOMO instead of 1 in LUMO (I underest, A overest, E underest)
- Gradient represents energy levels and are incorrect

(IMP) What is the solution to the long-range correlation error?
Include explicit non-local correlation or long-range dispersion terms, describing sum of all pairwise interactions as a function as 1/r^6 e.g. Many body dispersion/ vdWsurf
- What is an example of a meta-GGA
- TPSS
(IMP) How do 4th-rung hybrid functionals solve the localization problem in meta-GGAs? Use a sketch to support your answer
- Includes HF exchange, which strongly over localizes/stabilizes electrons, making graph more convex.