Lec 8- T cell development and immunity Flashcards
(33 cards)
T cell development
T cells are like B cells because
- They develop in bone marrow
- They produce receptors by gene reaarrangement
- They are taught to recognise self T cells are unlike B cells because
- They mature in the thymus
- Their receptors recognise peptide Ag in the context of MHC
T cells migrate to the thymus to mature
- T cell precursors travel from bone marrow to develop in the thymus
- Mature T cells leave the thymus and travel to secondary lymphoid tissues

Cellular organisation of the thymus
Cortex- cell density is far greater
- Cortical epithelial cells
- Thymocytes ( immature Tcells) were initially in bone marrow then enter medulla then to cortex then back to medulla for learning Medulla
- Medullary epithelial cells
- Macrophages
- Dendritic cells (APC

The thymus involutes with age (degrades)
- up to age of 10 your full size thymus
- After this time it reduces in size through a process called involution
- Tissue gets replaced by fat
- Older you are the worse it is
- It is hard to get vaccines to work in the over 50s age group because there is far less maturation of T cells
- For the vaccine to work you need a full response
There are 2 lineages of T cells from the same progenitor
- CD34 is an uncommitted progenitor cell –>
- Committed double- negative T cell progenitor
- If it goes the gamma delta route it will become a G/D cell
- If it goes down the a/b route (CD4 or 8)
- From this it can then decide weather to be a helper or cytotoxic T cell

2 checkpoints for T cells- TCR chain rearrangement checks
- Early development of alpha; Beta T cells
- Beta chain rearrangement comes first
- Successful alpha chain rearrangement ensure alphabet T cells are formed
- gamma;delta rearrangement happens at the same time as the beta chain
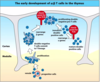
2 checkpoints for T cells- TCR chain rearrangement checks when it happens
- Progenitor cells
- Proliferation
- Double negative T cell commits to T lineage
- Rearrange Beta genes (checkpont for pre-TCR)
- Proliferating double-negative pre-T cells
- Immature double positive cells
- Alpha rearrangement (check point for TCR)
- Mature double positive cells

DP T cell screening- positive selection
- Positive selection of alphabet cells by cortical epithelial in the thymus
- Can we see self MHC- if we cannot see this means that the T cell cannot become activated meaning that it is useless
- This selection is done on weak or no binding, if this occurs then we go through the process to destroy the cell
- If moderate to strong binding to can progress on to proliferation (multiplication)
- MHC II on epithelial cells: exception to the rule (normally only on APC)

To help or to kill-MHC is the key to this decision
- Receptor binds self peptide; self MHC class I (i.e. if it binds well with CD8 co receptor) then this will progress to be a cytotoxic
- Receptor binds to self peptide; self MHC class II if there is strong binding with CD4 this will become helper cells
- The cells at this point have both CD4 and CD8 but after this process it will only have the co receptor with the strongest binding

DP T cell screening- negative selection
- Negative selection fo alpha;beta T cells by dendritic cells, macrophages and other cells in the thymus
- Is there strong self peptide binding
- If there is moderate or weak binding this is good and can be allowed to live
- If there is strong binding this suggests that the T cell will become activated when presented with self peptide, this T cell will be destroyed

A 3rd type of T cell
- Suppression of Auto-reactive T cells by regulatory T cells requires them to interact with the same APC
- Protects against imperfect selection of T cells
- Active suppression of autoreactive cells by cytokines
- IPEX- X-linked autoimmunity lacking regulatory T cells

T cell mediated immunity
-Priming- activation of naive T cells
+Primary immune response
-Development into effector cells T helper cells
+Help for macrophages (eating and killing)
+Help B cells (switch to plasma cells, class switching)
- Help for T cytotoxic cells
- Killer T cells
Immune response are concentrated in the secondary lymphoid tissue
- Wound with particles entering the body
- Dendritic cells take up the bacterial Ag in the skin and then move to lymphatic vessel
- They then enter the lymph node where dendritic cells bearing the Ag enter the draining lymph node, where they settle in the. T call area
- Any T cell that pass’s and can recognise will activate

Dendritic cells change function to be more effective
- Dendritic cells in peripheral tissue (lysosomal marker MHC II) they eat things
- Dendritic cells in the lymphatic circulation (MHC up regulation and surface expression)
- Dendritic cells in lymphoid tissue (will show the Ag of the pathogen to the T cells)
DC use many pathways to process and present Ag: routes of Ag processing and presentation by dendritic cells
Receptor mediated endocytosis
- extracellular bacteria; MHC class II using CD4 co receptor Macropinocytosis
- Extracellular bacteria, virus, Ag, pathogen particles; MHC II; CD4 Viral infection
- MHC I; CD8 Cross-presentation after phagocytosis uptake (virus has no time to infect before being engulfed
- MHC I; CD8 Transfer from incoming dendritic cells to resident dendritic cell
- Virus; MHC I; CD8

Naive T cells can enter lymph nodes from the blood

- T cells enter a lymph node across endothelial venules in the cortex (HEV)
- T cells monitor Ag Presented by macrophages and dendritic cells OR
- T cells that don’t encounter specific Ag leave the node in the efferent lymph
- T cells that encounter specific antigen proliferate and differentiate to effector cells
Naive T cells need co-stimulation to be activated
- The co-stimulatory molecule B7 on the dendritic cell binds CD28 on the naive T cell
- First signal is the normal MHC TCR with CD4
- There is a second signal that is needed and this is the B7 receptor on the dendritic cell which interacts with the CD28 receptor on the naive T cell
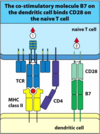
Co-stimulation prevents self-reactivity
- Co-stimulatory signal and specific signal means that the T cell is activated
- Specific signal alone (MHC with TCR and CD4) this will cause the T cell to become anergic (this means it will be killed off)
- If its just the Co-stimulatory signal alone- there is no effect on the T cell because it hasn’t seen its antigen

Helper T cells have different functions
- Naive CD4 T cell -Proliferating T cell
- Immature effector cell (this can either go the helper 1 or 2)
- T cell Helper 1:IL-2; IFN-gamma; macrophage activation, B cell activation and production of opsonising antibody such as IgG1
- T cell Helper 2 cell:IL-4;5; general activation of B cells to make antibodies
- Cytokine environment drives diffrerent development pathways
- Balance response
- Polarised response sometimes

Different help for humeral and cell- mediated immunity
- Most immune responses require a balance of Th1/Th2 cells
- Immune response can be polarised
- Positive feedback promotes polarisation
- Th1 response: cell-mediated immunity (effector cells)
- Th2 response: humoral immunity (Ab)
Polarisation can affect disease prognosis: leprosy
Th1- cell mediated response
- Activated macrophages
- Control of bacteria
- More limited disease
- Better of the 2 conditions Th2- cell humeral response
- Ab production
- No bacterial control
- Severe disseminated disease
- Affects bone, cartlidge, nerve and other parts of the body
- Best response will be a balance
Effector T cell activation
- Recognition- stimulation of T cell- complex Ag recognition AND B7;CD28
- Proliferation and differentiation- division and differentiation gives effector cells (release of IL-2)
- Effector function: once activated the T cell only needs the MHC (with Ag)
- TCR activation to kill the infected cell

3 types of effector T cell have complementary roles
CD8 T cells (cytotoxic)
- Virus infected cell
- T cell secretes effector molecules onto surface of target cell
- Cytotoxins: Perforin; granzymes; granulysin
- Cytokines: IFN-gamma; LT CD4 T cells (helper 1)
Ag presenting Macrophage
- T cell secretes effector molecules onto surface of target cell
- Cytokines: IFN-gamma; GM-CSF: TNF-a; LT; IL-3 Helper 2 cells
Ag presenting B cells
- T cell secretes effector molecules onto surface of target cell
- Cytokines: IL-4.5,10,13; TGF-Beta

Cytotoxic CD8 T cells kill selectively
- Collision and non-specific adhesion
- Specific recognition redistributes cytoskeleton and cytoplasmic components of T cells
- Release of lytic granules (degranulation) at site of cell contact