Hypothalamic-Pituitary- Adrenal Axis Flashcards
What are the six Anterior Pituitary hormones?
- ACTH: Adrenocorticotropic Hormone
- FSH: Follicle Stimulating Hormone
- TSH: Thyroid Stimulating Hormone
- PRL: Prolactin
- GH: Growth Hormone
- LH: Luteinizing hormone
What are the two Posterior Pituitary Hormones?
- production
- transport
- store
- ADH: Antidiuretic Hormone
- Oxytocin
- these are synthesised in neurones of the hypothalamus then converted to their active form in the posterior pituitary gland
- supraoptic nuclei
- periventricular nucleus
- inactive forms are transported from the nuclei along the hypothalamico-neurohypophyseal tract
- then stored in the posterior pituitary
Give an overview of Growth Hormone (GH)
- production
- control
- action/effect
- synthesised in the somatotroph cells of the anterior pituitary gland
- secretion is controlled by the hypothalamus
- GHRH (somatotropin) stimulates its secretion
- Growth hormone-releasing hormone
- has a greater role than GHRIH
- stimulated by decreased COH and fatty acids and increased amino acids
- GHRIH (somatostatin) inhibits is release
- Growth hormone-releasing Inhibiting hormone
- GHRH (somatotropin) stimulates its secretion
- acts via 2nd messenger produced in the liver in some tissues
- Insulin-like growth factor 1 and 2
- Primary effects: promote growth in adolescence by increasing protein synthesis and collagen deposition
- foetal growth is also dependent
Give an overview of Oxytocin
- production
- control
- action/effect
- works via IP3 to cause contraction of the smooth muscle of the genital tract and breast
- production increases during pregnancy
- a parallel increase in oxytocin activity is also seen
- secretion of oxytocin is achieved by stimulation of the genitals and nipples
- most important at parturition and lactation
- there is a delay between the start of suckling and milk let down
- Oxytocin is not necessary for the initiation of normal labour
- it can be administered to induce labour

Describe the cycle that leads to oxytocin release
- nipple stimulation
- stimulation of the cervix and vagina

Give an overview of prolactin
- release/ secretion
- control
- action
- secreted by lactotroph (mammotroph) cells in the anterior pituitary gland
- these cells increase during pregnancy and
- prolactin conc. increases during parturition initiating lactation
- secreted in both males and females
- secretion is controlled by
- Prolactin Inhibiting factor = DA
- TRH stimulates prolactin release
- secretion is stimulated by
- mild stress
- nipple stimulation
- coitus
- Action
- contain milk production in females
- maintenance of lactation depends on suckling
- prolactin with other hormones causes proliferation and differentiation of mammary tissue during pregnancy
- inhibits gonadotrophin release and/or response of the hormones to these trophic hormone (ovulation doesn’t occur during breastfeeding)

What are the actions of Prolactin?
(4)
- increase during pregnancy under the influence of oestrogen and progesterone to facilitate lobluloalveolar development of the breast
- causing milk secretion from the breast after oestrogen and progesterone priming
- inhibits GnRH secretion and antagonizes the action of gonadotropins in the ovaries, inhibiting ovulation
- hyperprolactinaemia in men is associated with impotence and hupogonadism
Give an overview of ADH
- release
- stimulation
- action/ receptors
- The hypothalamic nuclei that control fluid balance lie close to the nuclei that synthesise and secrete ADH.
- Stimuli for ADH release
- increased in plasma osmolarity (sensation of thirst)
- hypovolaemia - through stretch receptors in the CVS or angiotensin release
- ADH (vasopressin) receptors are all GPCRs
- V1A and V1B
- coupled to phospholipase C/ inositol triphosphate system
- oxytocin receptors also have GPCRs that are similar to ADH, therefore, ADH acts as a mild agonist at oxytocin receptors
- V2
- these stimulate AC which mediates the main response of ADH in the kidney on the basolateral membrane of the distal tubule and collecting duct
- increases the rate of insertion of aquaporins in the collecting duct in the kidneys
- V1A and V1B
Give an overview of the control of ADH
-
NSAIDs and carbamazepine increase vasopressin effects (Na+ retention)
- Aldosterone (mineralocorticoid) also causes water to be reabsorbed along with sodium.
- Lithium, colchicine and vinca alkaloids decrease vasopressin effects (Na+ excretion).

What clinical investigations are carried out?
- Presentation - Primary Or Secondary?
- Stimulate secretion? (ACTH) or Suppress secretion? (Dexamethasone)
- TSH & T4
- Cortisol
- LH & FSH
- A prolactin (PRL) test
- Testosterone / “Periods”
- After Biochemical Tests: Imaging (e.g. MRI)
- After Imaging: Visual Field Tests
- Bilateral hemianopsia (due to compression of optic chiasm)
Give an overview of the hypothalamus
- location
- inputs/outputs
- action/effect
- lies on either side of the third ventricle, below the thalamus, between the optic chiasm and the midbrain
- receives inputs from the limbic system and the retina
- contains neurons that are sensitive to changes in hormone levels, electrolytes and temperature
- efferent output to the autonomic nervous system
- has greater homeostasis of physiological systems:
- thirst, hunger, sodium and water balance, temp. regulation
- control of circadian and endocrine function
- formation of anterograde memories (with the limbic system)
- translation of response to emotional stimuli into endocrinological and autonomic responses
- has greater homeostasis of physiological systems:

What are Sellar masses and how do they present?
- a mass found in the sellar region in the brain this is composed of the
- bony sellaturcica
- pituitary gland
- adjacent structures
- present with neurological symptoms
- visual impairment: the most common is bitemporal hemianopsia
- __one or both eyes may be affected to varying degrees
- onset of the visual deficit is usually very gradual –> delayed ophthalmologic consultation
- diplopia
- headache: due to the expansion of the sella
- pituitary apoplexy: sudden haemorrhage into an adenoma –> sudden headaches and diplopia
- Cerebrospinal fluid rhinorrhea - inferior extension of the mass
- Parainaud syndrome: a constellation of neuro-ophthalmologic findings (most often paralysis of upward conjugate gaze),
- visual impairment: the most common is bitemporal hemianopsia
- discovered incidentally through MRI, usually with hormonal abnormalities
- hormone deficiencies are of gonadotropins –> hypogonadism
Go over the causes and prevalence of Sellar Masses
- types of adenomas
- differential
- 90% of cellar asses are pituitary adenomas
- these are benign tumours of the anterior pituitary that are neoplastic
- Prevalence of type of adenomas per 100,000
- All adenomas – 77.6
- Lactotroph adenomas – 44.4
- cause hyperprolactinemia –> hypogonadism in men and women
- Nonfunctioning adenomas – 22.2
- gonadotroph adenomas
- thyrotroph adenomas - may cause hyperthyroidism due to increased TSH
- Somatotroph adenomas – 8.6
- cause acromegaly due in increased GH secretion - the majority are clinically silent
- Corticotroph adenomas – 1.2
- cause Cushings disease, but majority remain clinically silent
- Pituitary hyperplasia may present as a sellar masses and be misdiagnosed as pituitary adenoma
Types of Pituitary hyperplasia
- Lactotroph hyperplasia during pregnancy.
- Thyrotroph and gonadotroph hyperplasia due to longstanding primary hypothyroidism and primary hypogonadism, respectively.
- Somatotroph hyperplasia due to ectopic secretion of growth hormone-releasing hormone, a rare condition.
How are Hypothalamic-pituitary hormones transported?
- Neurosecretory cells release peptidergic hormones (median eminence, hypothalamus) – transported in blood via the pituitary portal system.
- hormones also transport via axons from the Parvicellular neurosecretory cells
- Pituitary stalk & pituitary portal vessels pass down through the dura mater which roofs the pituitary fossa.

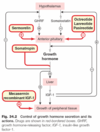
Review the Hypothalamic-pituitary-adrenal axis controls
(draw it out)


What type of imaging is this, label key features of the HP axis
- plane and weighting

- MRI scan, coronal plane, T1 weighted

What are the Hormones, Receptors & Enzymes of the Adrenal Cortex
- Adrenal cortex hormone production
- GLUCOCORTICOID
- CORTISOL
- MINERALOCORTICOID
- ALDOSTERONE (renin-angiotensin-aldosterone system)
- SEX STEROIDS
- ANDROGENS
- GLUCOCORTICOID
- Binding proteins:
- 90% cortisol bound to cortisol binding globulin (CBG)
- Receptors:
- Intracellular glucocorticoid and mineralocorticoid receptors (GR and MR)
- Enzymes:
- 11-b-hydroxysteroid dehydrogenase (11- b-HSD)
What are the effects of Glucocorticoids?
- Maintenance of homeostasis during stress
- e.g. haemorrhage, infection, anxiety
- Anti-inflammatory
- Energy balance/metabolism
- increase/ maintain normal [glucose]
- Formation of bone and cartilage
- Regulation of blood pressure
- Cognitive function, memory, conditioning
What are cortisol levels like across a day
- effect on the circadian rhythms
- rise during the early morning
- peak just prior to awakening
- fall during the day
- are low in the evening
- in preparation of sleep
Explain Ultradian Rhythms
- this is spontaneous pulsatile release of glucocorticoids during the day
- varying amplitudes
- amplitude decreases in the circadian trough
- associated with
- noise
- anticipatory stress
- response to an unintended stressor
What are the circulating androgens?
- DHEAS from the adrenal glands
- Androstenedione
- Testosterone
- converted to oestrogen by Aromatase
- Dihydrostesterone converted from testosterone by 5-alpha reductase

Explain the function of the 11-ß-HSD-1/2 enzyme
11-ß-HSD-2
- inactivates cortisol in the kidney, colon, sweat glands
- converts it to cortisone
- this allows aldosterone to bind to the mineralocorticoid receptor as they both have the same affinity
11-ß-HSD-1
- converts cortisone back to cortisol in the
- liver, adipose, CNS
- tissue specificity allows gating of GC access to nuclear receptors and amplification of GC signal in target cells

What is the impact of excess cortisol?
- Cushings syndrome
- weight gain
- central obesity
- hypertension
- Insulin resistance
- Neuropsychiatric problems
- Osteoporosis
What is the pathogenesis of Cushing’s syndrome?
- Excess cortisol due to
- Pituitary adenoma: ACTH-secreting cells
- Adrenal tumour: adenoma or carcinoma, neoplastic cells also secret cortisol
-
‘Ectopic ACTH’: carcinoid, paraneoplastic
- other non-adrenal cells producing cortisol
- Iatrogenic: steroid treatment
Clinical features of Cushing’s syndrome
- Central obesity with thin arms & legs
- Fat deposition over the upper back (‘buffalo hump’)
- Rounded ‘moon’ face
- Thin skin with easy bruising, pigmented striae
- Hirsutism
- Unwanted, excessive hair growth in women either on face, chest or back.
Causing
- Hypertension
- Diabetes
- Psychiatric manifestations
- Osteoporosis
What is the impact of insufficient Cortisol?
- Addison’s disease
- gradually falls off in general health at length they gradually sink and expires
- becomes languid & weak
- indisposed to either bodily or mental exertion
- the body wastes
- slight pain is referred to the stomach
- there is occasionally actual vomiting
- discolouration of the skin
What is the pathogenesis of Addison’s disease?
- Primary adrenal insufficiency ‘Addison’s disease’
- Usually autoimmune in UK
- Rare causes include metastases or TB
- decreased Production of all adrenocortical hormones
- Other causes of hypoadrenalism
- Secondary to pituitary disease (rare)
- ‘Iatrogenic’
- patients on high dose, long term steroid Rx, which is suddenly stopped at a time of stress
What are the clinical features of Addison’s disease?
- Malaise, weakness, anorexia, weight loss
- Increased skin pigmentation:
- knuckles, palmar creases, around / inside the mouth,
- pressure areas, scars
- Hypotension / postural hypotension
- Hypoglycaemia
What is Autoimmune Polyendocrine Syndrome?
- Types
- having multiple autoimmune endocrine dysfunctions
-
Type 1
- rare, onset in infancy
- AIRE gene (Ar)
- common phenotype:
- Addison’s disease
- Hypoparathyroidism
- Candidiasis
-
Type 2
- more common- infancy to adulthood
- polygenic cause
- common phenotype
- Addison’s disease
- Type 1 diabetes
- Autoimmune thyroid disease
What autoimmune conditions might occur together in APS?
- Type 1 diabetes
- Autoimmune thyroid disease (hypo- or hyper-)
- Also gestational / post-partum thyroiditis
- Coeliac disease
- Addison’s disease
- Pernicious anaemia
- Alopecia
- Vitiligo
- Hepatitis
- Premature ovarian failure
- Myasthenia gravis
What are the clinical implications of those with Autoimmune Polyendocrine Syndromes?
- actions to take
- High index of suspicion for additional autoimmune endocrine disorders
- T1 DM with fatigue, weight loss & hypos:
- ? Addisons disease
- T1 DM with non-specific GI symptoms / diarrhoea:
- ? Coeliac disease
- T1 DM with fatigue, weight loss & hypos:
- Consider screening in patients with T1 DM and/or Addison’s disease
- Coeliac screen
- Thyroid function tests (esp in pregnancy / post-partum)
How is the HPAA assessed?
- Basal function tests through
- Blood
- cortisol
- ACTH
- timing
- Urine
- cortisol
- 24 hr collection
- Saliva
- cortisol
- timing
- Blood
- Dynamic Tests
- stimulated
- ACTH
- CRH
- stress - hypoglycaemia
- suppressed
- Dexamethasone: synthetic glucocorticoid
- stimulated
How would too much cortisol be recognised through HPAA assessments?
- 24 hour urinary free cortisol
- ‘AREA UNDER THE CURVE’
- Midnight cortisol (blood / saliva)
- ‘TROUGH’
- 9 a.m. ACTH (with paired cortisol)
- PITUITARY / ADRENAL / ECTOPIC?
- NEGATIVE FEEDBACK AT PITUITARY
- PITUITARY / ADRENAL / ECTOPIC?
- DEXAMETHASONE SUPPRESSION
- Sensitivity to GC negative feedback at pituitary
How would too little cortisol be recognised through HPAA assessments?
- 9 a.m. cortisol
- ‘PEAK’
- SynACTHen test
- Adrenal response to ACTH
- Trophic effect ACTH on adrenals
- Adrenal response to ACTH
- Insulin tolerance test
- Response to hypoglycaemic stress
- Can be dangerous!
- Response to hypoglycaemic stress
- U & E (¯Na, K) in Addison’s disease
- Due to mineralocorticoid deficiency
- Can measure renin & aldosterone concentrations
- decreased blood glucose
What golden rules should be followed when assessing the HPAA?
- Never start investigating a patient for an endocrine condition unless their symptoms & signs suggest they may have it!
- Risk of false-positive results
- Never image any endocrine gland until you have established the diagnosis biochemically!
- Risk of discovering ‘incidentalomas’
What imaging should be carried out when assessing the HPAA?
- after confirming Cushing’s syndrome, consider
- CXR
- MRI pituitary
- CT adrenal
- Rarely image Addison’s disease patients unless concerned about TB/ metastatic cancer
What is the medical management for Cushing’s syndrome?
- Surgical (depending on the cause)
- Transsphenoidal adenectomy
- Adrenalectomy
- Pituitary radiatherapy
- 131I
What is the medical management of Addison’s disease?
- Glucocorticoid replacement therapy
- usually, hydrocortisone (sometimes prednisolone)
- needs to be increased to cover ‘stresses’ (intercurrent illnesses like flu)
- recommendations may vary for operations and post-op period
- Mineralocorticoid replacement therapy for those with primary adrenal insufficiency
- fludrocortisone
- additional hormone replacement therapy for those with secondary adrenal insufficiency
- patients need IV/IM steroid if unable to do so orally
- vomiting/ NBM
What is the effect of long-term high dose steroid treatment on patients?
- endongenous suppression of adrenal function
- They may not mount an adequate ‘stress response’.
- Their steroid treatment should not be stopped suddenly.
- If they need a major procedure / an operation, they require increased steroid cover as described.
- They should be given a ‘Steroid Treatment Card’ to remind them (& their doctors) about this.



