Heteroaromatics Flashcards
Show the enol and enolate form of a carbonyl.

Draw the formation of an imine from a carbonyl.

Draw the aldol and mannich reactions. (enol Nu)

Draw and describe the conditions of a Michael reaction.

Draw the general mechanism of the Friedel-Crafts reaction.

What are the methods of producing 1,3, 1,4 and 1,5 dicarbonyls?
1,3: Enolate and electrophilic carbonyl with LG
1,4: Enolate and carbonyl with an α-bromine LG
1,5: Michael reaction with soft carbonyl nucleophile
Draw the following molecules: pyridine, pyrrole, furan and thiophene.

Draw the following molecules: imidazole, oxazole, thiazole, quinoline, isoquinoline and indole.

What are the differences between benzene and pyridine?
The electronegativity of the nitrogen makes the ring inductively π-defficient compared to benzene. This deshields the hydrogen closest to the N.
The lone pair of N points out of the ring so it acts as a nucleophile and a weak base.
How can pyridine be used for esterification reactions with an acid chloride? Why is this useful?
The pyridine substitutes at a carbonyl, removing a chloride. Pyridine then acts as a leaving group when an alcohol attacks the carbonyl. The pyridine then acts as a base and cleans up the HCl produced.
Describe how pyridines undergo electrophilic aromatic substitution. Draw relevant mechanisms.
Pyridines by themselves are not good at electrophilic aromatic substitution as: electrophiles react at N, the ring is π defficient and the N destabilises the intermediate.
However a pyridine N-oxide, where the pyridine is bonded to an O-, can undergo a substitution. The oxygen adds electron density, making the pyridine nucleophilic.

For pyridine, explain where nucleophilic aromatic substitution will occur.
At C2 or C4 but not C3 beacause it doesn’t have a resonance form with the negative charge on the nitrogen. This is analogous to enones (carbonyl and alkene).
What is the Chinchibabin substitution reaction? Draw the mechanism and give possible reagents of varying strengths.
This is also possible with organolithiums and Grignards. The reaction can also occur with weaker nucleophiles if a better LG is present such as Cl-.

How can nucleophilic aromatic substutions occur using pyridine N-oxides? Draw the mechanism. What is the driving force of the reaction?
PO2Cl is a very good leaving group and it is very favourable to form the oxygen double bond.

What is notable about the reactivity of 2 and 4-methyl pyridines?
They undergo elimination of a methyl hydrogen using LDA to form a double bond off the ring and a negative charge on the nitrogen. This can then react as a nucleophile, similar to how a enolate would react.
Describe the basic RSA of pyridines and draw how they can be synthesised.
The pyridine contains groups that appear to look like an enamine and an imine. Therefore they can be synthesised from a 1,5-dicarbonyl and an amine which is in turn synthesised from a carbonyl Michael reaction.

Draw a mechanism for the Hantzch synthesis and give a summery of the basic steps.
Form an imine from one carbonyl, the other two carbonyls undergo an aldol reaction which is then attacked by the imine, which then ring closes.

Describe the structure of pyrrole.
The 5 atom ring has a nitrogen with its lone pair delocalised into the ring giving it the same number of π electrons as benzene. This makes the ring π-excessive (greater electron density than benzene) which is reflected in lower chemical shifts.
What strength base can deprotonate pyrrole?
Only strong bases such as NaH since the hydrogens are all orthogonal to the delocalised p orbitals.
How and where does pyrrole react with electrophiles?
It reacts at C2 by aromatic substitution since this gives the ring the most resonance structures. This reaction is fast since the ring is π-excessive meaning activating agents aren’t as important.
Draw the mechanism of the Vilsmeier formylation with pyrrole to introduce a COR group.
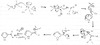
How can the Mannich reaction be used to add poor nucleophiles onto a pyrrole? Draw the mechanism.

How can pyrroles be made into good nucleophiles? How does this compare for furan and thiophene?
A strong base can deprotonate at the nitrogen which is then reacted with an electrophile such as MeI to form the substituted nitrogen.
BuLi can then be used to deprotonate at C2 (C2 is favoured due to nitrogen -I effects) which can then react with various electrophiles.
For furan and thiophene the same proccess can occur, except the heteroatom doesn’t need deprotonating first.
Describe how pyrrole can be anaylsed to construct a synthesis. Draw the mechanism of the Hantzch synthesis.
There is an enamine type group so the cyclisation can occur from a 1,4-carbonyl-imine where the iminium attacks the carbonyl. The 1,4 structure is formed from a substitution at the alpha carbonyl position.
















