Formulas Flashcards
How to calculate molar mass, aka molecular mass or atomic mass.

How to convert from grams to moles?

How to convert grams to molecules?

Conversion Shortcuts:
- Moles to Atoms / molecules
- Atoms / molecules to moles
- Moles to grams
- Grams to Moles

How to calculate empirical formula

How to balance a chemical equation

How to give IUPAC name for a compound
First, determine if the compound is:
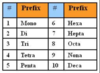
- Ionic = Metal + Nonmetal (cation and anion). Always balance out charges.
- Molecular = Multiple non metals = use prefixes and suffixes.
How to calculate volume
General formula:
Density = mass/volume
Rearranged: Volume = mass/density
How to convert mass to moles
Just divide by atomic weight!

How to find limiting reagent

How to calculate molarity

Stoichiometry Template

Stoichiometry Made Easy - Cheat to Find How many grams of x will be produced x? In this example, how many grams of ammonia will be produced?
- Always start by balancing equation
- Now, put an x in front of every compound
- Place molecular weight of each compound in front of each x.
- Solve for x.

How to approach Stoichimoetric Calculations - from TRU



