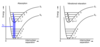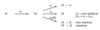Excited states and Photochemistry Flashcards
What is photochemistry and where does it take place?
Chemistry initiated by light
Takes place in the excikted state created by absorbtion of a photon with energy hv
When does an elevctronic transition take place?
- When an electron moves from one MO to another:
the electron density is redistributed
the bonding and structure chage
photochemical reactions can be quite different from thermal reactions of the same molecule
- Typically indiced by UV light
wavelengths in the range of λ = 200-800 nm
absorbers called chromophores
Equations for:
energy of one photon
energy of a mol of photons
wavenumber converting m - cm and m-1 - cm-1
Realtionship between energy, frequency, wavenumber and wavelength

Units and symbols of frequency, wavenumber, wavelength, energy (of one photon and a mol of photons), speed of light, planck’s constant, avogadro’s constant

What is the beer lambert law and what are the units of the components

Typical long-wavelength absorption properties of saturated and unsaturated systems and some typical transitions
saturated systems: usually absorb in the vacuum UV
unsaturated systems: increasing conjugation shifts the absorbtion to longer λ
σ—–>σ*
π—–>π*
n—–>π*
Transitions invloving σ* prbitals usually give higher bands (λ<200 nm)
Describe the states a molecule can be in
Ground state:
the lowest energy electronic state
electrons generally occupy bonding MOs and in some cases non bonding orbitals
Excited state:
Photon absorbtion induces an electronic transtion where an electron is excited to a higher energy level usually an antibonding MO
Explain and draw the different types of transition that can occur between MOs
When electrons occupy the same MOs but with different spins different excited states can be achieved.
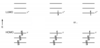
What is multiplicity
Multiplicuty is used to describe the electron spin properties of a state
Defined as 2S+1
When an electron retains its spin on transtion multiplicity remains constant
When an excited state has a multiplicity of 1 it is called a singlet transition, when a transition has a multiplicity of 3 it is called a triplet transition
Give the labels for singlet and triplet transitions and the ground and excited state
How many triplet states are there?
Singlet = S
Triplet = T
Ground = 0
Excited = 1,2,3…
Each excited singlet state has an equivalent triplet state at lower energy (hunds rule states that subshells will preferentially fill singly beofre doubly).
There is no T0 state (Pauli exclusion principle says that 1 orbital cannot be doubly occupied with 2 electrons of the same spin).
Describe the rotational and vibrational degrees of freedom of atoms and molecules?
Atoms: no rotational or vibrational degrees of freedom
electronic energy levels are clearly defined
UV-visible spectra has sharp lines
Molecules: has rotational and vibrational degrees of freedom
eavh electronic state has many energy levels
UV-visible spectra has many lines or braod bands
Describe the Born-Oppenheimer approximation
states that the elctronic, vibrational and rotational properties are considered to be independent of each other

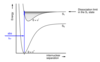
Where do electronic and vibrational transitions usually occur from and to?
Electronic trnsitions occur from the ground state (S0) to the first excited state (S1) when the longest wavelenght absorption arises
From v’’ = 0 in the S0 state (Boltzman states this is the most populated)
To: several v’ levels in the s1 state

Describe typical rotational energy transitions
Transition genrally occur from several J’’ rotational levels in the v’’ = 0 level (as Boltzman states that many J levels are populated) to several J’ leve;s in each v’ level in S1

Describe what typical spectra can look like
Rotational fine structure is seen from gases at high spectroscopic resolution
Vibrational structure seen from gases and liquids/solids in some cases but peak can be broad - particularly in polar solvents due to strong interations
What determines the required energy of a photon for a transition and the probability of the transition?
The energy of the photon (wavelength of light) is determined by the energy gap between the ground and excited states
The probability (stregth of absorption coeffiecient) is determined by selection rules
What is the overall selection rule for an electronuc transition?
The elctric field of the light must cause a displacement if charge giving a transition dipole moment (TM)
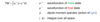
How do you apply the born oppenheimer approximation to the overall selection rule?
separate thr electronic anf vibrational wavefunction:
ψ = ψe . ψv
Separate ψe into electron spin (S) and orbital wavefuntions:
ψ = S . φ . ψv
Substitute into the original TM equation noting that the TM operator (μ)only acts on the orbital wavefunction
Tis gives three separate selection rukles: spin (S), orbital (φ) and vibrattion (ψv)
IF any of the integrals are 0 the TM is 0 and the transition if disallowed

What is the spin selection rule?
An electron has two possible spin states:
spin up (α) and spin down (β)
When the electron retains its spin on transition S’* and S’’ are both Sα or Sβ - The integral is normalised to and therefore = 1
If the spin changes on transition S’* = S’*α and S’’ = S’‘β or vice versa and the integral is orthogonal and therefore = 0
Rule: the electron must retain its spin on transition for the transition to be allowed (triplet excited state cannot be created efficiently by absorption.
Explain the orbital selection rule
The rbiotal selection rule has two parts:
symmetry and spatial
Symmetry rule: The orbital part of the transition can be split into three parts:
TMorbital = TMx + TMy + TMz
All three components must be 0 for the transition to be forbidden
Spatial: If the initial and final orbitals occupy a region of space that is similar the transition is allowed
Describe how to determine which transiotion are symmetry allowed
Deduce the point group
Deduce the symmetries (irreducibles) of the initial and final states (Γi and Γf)
Deduce the symmetries of the x, y and z components of the dipole moment operator (read from character table) (Γx, Γy, Γz)
Deduce three direct products
Γf x Γx x Γi - repeat for y and z
If any direct products gives totally symmetric represention (A1/Ag) the transition is symmetry allowed
Which transitions are allowed and forbidden under the spatial selection rule and what can affect the size of μ
π —–> π* have good spatial overlap and therefore are spatially allowed
n —-> π* occupy different regions of space and therefore are spatially forbidden
If there is a l;arge displacement of charge μ will be large
For a harmonic oscillator give the most probable geometries for v = 0 and v >> 0and give the most probable transitions
v = 0: most probably geometry (most probable point of finding particle) is at equilibrium geometry
When v >> 0 the most probably geometry is at the turning points
electronic transitions occur mainly from v’’ = 0 in the S0 state to v’ levels that give large vibrational overlap
What is the Franck-Codon Principal?
electrons move faster than nuclei because of their relative masses
There is no change in the position of atoms during a transition
Any change in geometry occurs after the transition.