Exam 2 Flashcards
Central Dogma
Crick
DNA –transcription–> RNA –translation–> protein
gene structure
promoter: initiates expression
5’UTR: regulates gene expression
open reading frame: RNA information (introns and axons)

mRNA: location and function
functions in nucleus, migrates to ribsomes
carries DNA sequence info to ribosomes
tRNA: location and function
functions in cytoplasm
provides linkage b/w mRNA and AAs, transfers AAs to ribosomes
ribosomal RNA/rRNA: location and function
functions in cytoplasm
structural component of ribosomes
post transcription regulation: how are eukaryotic genes segmented?
enzymes cut out introns
exons spliced together to make mRNA
more than 90% of pre-mRNA is destroyed (introns)
alternative splicing
single gene can code for multiple proteins by mixing and matching exons
mutations occurs as ____ and cause changes in the _____
random chance events, DNA sequence
if mutations occur in ____, then ____
gametes, they can be passed onto offspring
mutations may be caused by ____
exposure to toxins or radiation (mutagens)
others lead to variations that are good for organism to adapt to environment
two kinds of gene mutations
- Gene mutations:
- Single gene
- Substitution, stop, inversion, insertion, and deletion
- Chromosomal mutations:
- Abnormal chromosome structure (affects multiple genes)
- Substitution, stop, inversion, insertion, deletion, and translocation
down syndrome
trisomy 21 (extra 21 chromosome)
alters child’s phenotype - characteristic facial features, short stature
usually some mental retardation
major types of genetic disordesr
- Autosomal
- single genes
- multiple genes
- Sex-linked
- Chromosome abnormalities (eg down syndrome)
Recessive traits vs dominant
recessive: normally loss-of-function mutation
dominant: normally gain-of-function mutation
autosomal genetic disorders + examples
caused by alleles on autosomes (chromosomes other than sex chromosomes)
most are recessive (need two alleles)
carriers ex (have 1 recessive) : CF, sickle cell
dominant ex: huntingtons (only need 1 allele)
sex linked genetic disorders
common Y-linked disorder: male infertility
X-linked recessive disorders (most common in males): hemophilia, color blindness, muscular dystrophy, fragile-X syndrome
2 ways of identifying disease mutations
- Linkage analysis:
- Data collected for family members
- Good for rare disorders
- Genome-wide association studies (GWAS)
- Data colleced for unrelated individuals
- Good for common diseases
autosomal dominant vs recessive, how to identify
dominant: affected parents have affected children
how to identify x linked dominant vs recessive
- x-linked dominant:
- affected mother: either son or daughter can be affected
- affected father: only can pass to daughter, but not son
- x linked recessive:
- female carrier: only son can be affected
SNP vs microsatellite
single nucleotide polymorphyism: just one base changed at specific point
microsatellite: series of bases repeated several times
types of SNPs: figure
synonymous: single nucleotide change does not change AA sequence
missense: one AA change

GWAS has been applied to ___, and what does graph mean
alzheimers, autism, schizophrenia

which animal models easy to house large numbers
best (a): C. elegans (worm), fruit fly, zebra fish
also good (b): mice
which animals have large number of offspring
best (a): C. elegans, fruit fly, zebra fish
also good (b): mouse
animals that are good genetic tools
best (a): C elegans, fruit fly, mice
also good (b): zebra fish, monkey
which animals have short generation time
best (a): C elegans, fruit fly
also good (b): zebra fish, mice
which animals have transparancy
best (a): zebra fish
also good (b): C elegans, fruit fly
which aniamals have similarity in organization to human CNS
mice and monkeys
animals good for studying single neuron vs cognitive learning/memory
single neuron: C elegans
cognitive learning/memory: monkey
Morgan nobel prize (193)
studied sex limited inheritance in flies
discovered the role that chromosome in heredity
what are UAS-GAL4 and FRT-FLP systems used for
UAS-GAL4: used to manipulate gene expression
FRT-FLP: used to make mosaic clones
forward vs reverse genetics
forward genetics: identify interesting phenotype, then discover the genes defective in the mutants
reverse genetics: alter known gene and see how phenotype changes
forward genetics: how are random mutated genes introduced
- Chemical: ethyl methyl sulfonate (EMS)
- Point mutations, tedious mapping process
- Radiation: X-ray or gamma rays
- Chromosome deletions/rearrangement, inefficient
- Transposons (P element)
- trigger DNA insertion, easy mapping
classic F3 screen for recessive mutations
- Diploid screen for dominant mutations (F1 screen)
- Diploid screen for recessive mutations (F2 screen)
- Diploid screen for recessive mutations (specific locus screen)
nobel prize in physiology or medicine, 1995
Lewis, Nusslein-Volhard, Wieschaus
nobel prize for discoveries on genetic control of early embryonic development
sonic hedgehog
can be loss of function (mutant embryo much smaller) or gain of function (double wing, double fingers, double head in snakes)
UAS-GAL4
- used to manipulate gene expression in flies
- derived from yeast
- GAL4 = transciption factor
- UAS = upstream activating sequence
- when GAL4 bind to UAS, triggers gene expression
mosaic analyses definition
express mutation in only some daughter cells
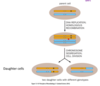
FRT-FLP system
- used to label cells via mosaic techniques
- site-directed chromosomal recombination
- FRT = specific DNA sequence
- FLP = flippase or FLP recombinase
- randomly label some daughter cell, but not all cells
general approach to doing reverse genetic manipulation
- Build a vector (brings foreign DNA into cell)
- Gene targeting vector for homologous recombination
- Use vector to transfect cells of interest and express foreign protein
- expression may be transient or stable
- transient expression may be inducible or repressible
- expression may be transient or stable
- Assay structure or function
what is addgene
website that’s a database of vectors
reverse genetics: building an appropriate construct (what are the pieces)
GFP example
want to construct pcDNA3.1-GFP:
- CMV promotor (generic promoter to express genes in all cell types)
- GFP gene
- Stop sequence: bGH poly(A) signals
- pcDNA3.1-GFP for subcloning
reverse genetics: transfection involves what
GFP example
- can transfect into cell lines/cultured neurons (in vitro):
- calcium phosphate transfection: carries DNA into the nucleus, not high efficiency (not many neurons are transfected)
- lipid transfection: higher efficiency
- electroporation: makes pores in membrane for DNA to enter; highest efficiency
- can also transfect in vivo:
- electroporation in utero, then inject construct
- low efficiency in vivo
transgeneic animals
- integrate foreign DNA randomly
- expression is controlled bc of endogenous sequences + inserted DNA has promoter sequence and coding sequence of gene of interest
- possible in any animal
knockout or knockin animals
- Replace endogenous gene w/ a version that cannot function (knockout, KO) or functions differently (knockin, KI)
- Expression controlled by endogenous promoter
- Works via homologous recombination
- efficient only in flies and mice
how to make a transgenic mouse
make a transgenic vector containing promoter, gene of interest, and stop coding sequence; then integrate into genome
- Random insertion
- Unknown copy number (may insert 1 or more copies)
how to make a transgenic mouse: method 1
- Microinjection of foreign DNA into fertilized oocytes (include promoter)
- Implant into foster mother
- Screen offspring by southern blotting or PCR
potential problems that can arise when using transgenic animals
- lack of control over where DNA integrates
- sometimes transgene is silenced by local elements)
- sometimes transgene insertion can cause an unintended mutation
- sometimes expression of phenotypes are not directly a result of the transgene
4 key steps to making KI and KO mice through homologous recombination
isolate and characterize gene of interest
generate a targeting vector
perform homologous recombination in embryonic stem cells
generate mice from the ES cells that express the modified gene
how to make a KO mouse: targeting and ES cell screening
- Design construct w/ stop codon to disable gene of interest
- Replace endogenous copy of gene by homologous recombination
- Transfect mouse ES cells (pluripotent) w/ KO construct
- Add positive selection marker (usually neomycin resistance) to select for cells that have taken up DNA (all cells w/o neo cassette will die)
- Add negative selection marker (construct w/ toxin; if randomly inserted, toxin will kill cells)
how to make a KO mouse: what do you do after developing your construct
inject ES cells into a mouse blastocyst
how to make a KO mouse: what do you do after you inject ES cells into a mouse blastocyst
transplant blastocyst into pseudopregnant mother
generates chimeric mice (usually use ES cells from a mouse line w/ different coat color than blastocyst to identify chimeras that have both colors)
cross best chimeric mice w/ WT mice to get germline transmission
identify gene targeting by southern blotting or PCR
summary steps of making KO mouse
- Make targeting construct
- ES cell transfection
- Positive (neo resistance) and negative (HSV-tk) screening
- Inject ES cells into blastocysts
- Implant blastocysts into foster mother
- Birth and breeding of chimeric mice
Nobel prize for creation of knockout mice
Capecchi, Evans, Smithies
developed principles for introducing gene modifications in mice by using ES cells
KI mice
directly overexpress GFP by replacing endogeneous genes
use positive (neo) andnegative (diptheria toxin, directly kills cells after expression) screen
express GFP w/ control of Gfib promoter
if ES cells are derived from mouse strain 129/SV with agouti coat and
the recipient blastocysts are derived from the mouse strain CD1 with white
coat, which offspring in the picture is the best chimeric mice?
mouse with most agouti (brown) coating
originates more from strain 129/SV (ES cells), so higher chance of having gene
common problems of making KO or KI mouse
- many KOs have no phenotype
- many transgenic, KO, or KO modifications are lethal during early development
name the different promotoers and where they are expressed
- GFAP promoter: expression only in astrocytes
- MBP promoter: oligodendrocytes
- GAD67 promoter: GABA expression neurons
- CAMKIIα promoter: glutamatergic neurons of forebrain (hippocampus)
- ChAT promoter: motor neurons
example of how we can selectively knockin a gene in motor neurons
Conditional knockin mice:
- Use ChAT promoter with Cre recombinase
- Cross Lox-Stop-Lox transgenic mouse with ChAT Cre mouse
- Cross triggers deletion of stop cassette only in cells w/ Cre, then promoter can drive transgene expression just in motor neurons

example of how we can selective knockout a gene in motor neurons
Conditional knockout mice:
- Use ChAT promoter with Cre recombinase
- Cross floxed mouse (gene sandwiched b/w two LoxP sites) with ChAT Cre mouse
- Cross triggers deletion of gene only in cells w/ Cre

spatial control in fruit flies example (intersectional approach): GAL4 and FLP systems
- GAL4 activates UAS system (but prevented by stop cassette flanked by FRT)
- Use FLP to remove stop cassette to trigger gene expression
- Use two different promoters for GAL4 and FLP; only when both promoters are active is the gene expressed (AND logic)

spatial control in fruit flies example (intersectional approach): GAL4 and GAL80 systems
- GAL80 prevents GAL4 from interacting with UAS
- only cells that express promoter A but not B can express the gene (NAND logic)

FRT-Flp system
Flp: catalyzes DNA recombination
FRT: 34bp DNA sequence
naturally occurring in yeast
- if FRT oriented in same direction: delete DNA in b/w
- if FRT oriented in different direction: invert DNA
- if FRT on two diff homologous chromosomes: translocation of arms of chromosomes