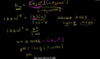EK Chem Ch7 Acid, Bases,Titration Flashcards
Titration
Titration is the process of finding / to determine the concentration of an unknown solution (the analyte) by reacting it with a solution of known concentration (the titrant). A solution of known concenteartion is used to determine the concentration of an unknown solution (titrand or analyte)
The analyte is generally placed in an Erlenmeyer flask, while the titrant is placed in a burette so that the volume of solution added can be monitored.
The titrant is added to the analyte until the endpoint is reached. Calculations are then performed to find the unknown concentration of the analyte. Titrations are typically performed for acid/base reactions but are not limited to them.
equivalence point titration
At equivalence points during the titration, the number of acid or base groups added to the solution is equivalent to the number of base/acid groups in the original unknown solution. We can calculate our unknown concentration or volume using the formula NaVa = NbVb, where N and V are the normality (mol/L) and volume of the acidic and basic solutions, respectively. It is important to convert from molarity (M) to normality (N) for polyprotic acids and polyvalent bases.
flat regions of titration curves
=represent buffering solutions (a roughly equal mix of an acid/base and its conjugate), while the steep, near-vertical sections of the curve contain equivalence point(s), which indicate that enough of the titrant has been added to completely remove one equivalent (acid or base group) from each of the original molecules in the unknown solution. Species with multiple acid or base groups (e.g. H3PO4 or Ca(OH)2) will have multiple equivalence points during the titration.
end point of titration
The final key point of any titration is the endpoint. To be successful, there must be some method for observing the endpoint of the reaction. The type of titration reaction that is being used will determine the method used for observing the endpoint. For example, in an acid-base titration, a specific pH value will be the endpoint (monitored by color-changing indicators), while for precipitation reactions, the endpoint is realized by the appearance of a precipitate. Regardless of the details of the reaction involved, the goal of titrations is always to use known volumes/concentrations to determine unknown volumes/concentrations.
Based on Figure 2, what is the approximate pKa of the carboxylic acid group in glycine?
answer is 2.4, In the Henderson-Hasselbalch equation, pH = pKa + log[B]/[A], where B is the conjugate base of the weak acid (represented by A). If [B] = [A], the pH will equal the pKa. This occurs for the first plateau in the titration curve for glycine shown in Figure 2. Estimating the pH at the middle of the first plateau gives a value closest to 2.4, as shown below.
Ka
Acidic species can be strong (for the exam, assume that strong acids completely dissociate in water) or weak. When an acid dissociates, it releases a proton to make the surrounding solution acidic. However, weak acids only partially dissociate and at equilibrium coexist in a deprotonated state (A-) and a protonated state (HA), according to the equation HA ⇌ H+ + A-. The concentration ratio of products and reactants is constant given fixed conditions and is called the acid dissociation constant (Ka). Ka is defined by the equation below. Ka = [H+][A-]/[HA]
ACID IONIZATION CONSTANT or ACID DISSOCIATION CONSTANT
Ka equation
Ka = [H+][A-]/[HA] The square brackets indicate the concentration of the respective aqueous species. Ka expresses how easily an acid releases a proton (i.e. its strength). In addition, this equation shows how the dissociation state of weak acids vary according to the [H+] level in the solution. A commonly tested family of acids on the MCAT are carboxylic acids (those containing –COOH), such as lactic acid and amino acids, which normally have a Ka of approximately 10-3 to 10-6. As this shows, expressing acidity in terms of the Ka constant alone involves inconvenient numbers that are not very intuitive.
pka
Therefore, pKa was introduced as an index to express the acidity of weak acids, where pKa = -logKa. For example, the Ka values for lactic acid (HC3H5O3) and nitrous acid (HNO2) are 8.3 × 10-4 and 4.1 × 10-4, respectively. The pKa values for these acids are 3.1 and 3.4, respectively, which are simpler expressions that are easier to understand and compare. The smaller the pKa value, the stronger the acid (since as X increases, pX decreases). Therefore, the pKa values above tell us that lactic acid is a stronger acid than nitrous acid.
pka for diprotic
For diprotic amino acids (i.e. those amino acids for which the side chain is neither acidic nor basic), the pI can straightforwardly be captured as the average of the two pKa values.
pka for triprotic
For triprotic amino acids (i.e. those that have an acidic or basic side chain), the pI can be obtained by averaging the two acidic pKa values for acidic amino acids or the two basic pKa values for basic amino acids. Another way of saying this is that for acidic amino acids, the pI is the average of the two lowest pKa values; for basic amino acids, the pI is the average of the two highest pKa values. Be careful to avoid averaging all three values given; the pI is always the average of two, not three, pKa values.
buffer 1
Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions or hydroxide ions are added or removed to solution. An acid-base buffer typically consists of a weak acid and its conjugate base. ***The most important buffer to know for the MCAT is the bicarbonate buffer system, which is shown below. H2O (aq) + CO2 (g) ⇌ H2CO3 (aq) ⇌ H+ (aq) + HCO3− (aq) Buffers resist pH changes best when the pH values are at or near the pKa value for the acid/base used, because that is when the conjugate acid and base have equal concentrations. Optimal buffering occurs when the pH is within approximately 1 pH unit from the pKa value of the system. The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation: pH = pKa + log [conjugate base] / [acid].
bicarbonate buffer system
***The most important buffer to know for the MCAT is the bicarbonate buffer system, which is shown below. H2O (aq) + CO2 (g) ⇌ H2CO3 (aq) ⇌ H+ (aq) + HCO3− (aq) Carbonic acid (H2CO3) has the conjugate base of HCO3−. Buffers work because the concentrations of the weak acid and its salt are large compared to the number of protons or hydroxide ions added or removed. When protons are added to the solution from an external source, some of the bicarbonate in the buffer is converted to carbonic acid, using up the protons added; when hydroxide ions are added to the solution, protons are dissociated from some of the carbonic acid in the buffer, converting it to bicarbonate and replacing the protons lost.
bicarbonate buffer system 2
On Test Day, you may see the bicarbonate buffer concept tested from a chemical, biochemical, or biological perspective. For example, hyperventilation (rapid shallow breathing) results in excess CO2 being expelled from the blood, causing the pH to rise. In response, the buffer needs to release more H+ to lower the pH back to physiological norms. An additional fact to be aware of is that other mechanisms in the body are also used to regulate pH, since carbonic acid works best at a pH below physiological conditions, because its pKa1 (pKa1 = 6.3, pKa2 = 10.3) is much lower than the normal pH of blood (7.4).
What is the approximate pH of a saturated aqueous solution of hydrochloric acid whose molarity is 10.6 M?
= -1 Hydrochloric acid is a strong acid and completely dissociates in aqueous solution. In this solution, the hydronium ion concentration is 10.6 M, which can be approximated as 10 M to make the math easier. The pH is the -log of the hydronium ion concentration: -log[10] = -log[101] = -1. While the typical pH range is normally thought of as ranging from 0 to 14, if the concentration of hydronium ion is greater than 1 M, negative pH values are possible. It is also possible to have pH values greater than 14, i.e. if the hydroxide concentration is greater than 1 M.
How acidic or basic a solution is can be expressed in terms of pH or pOH……..
are defined as follows: pH = −log [H+] and pOH = −log [OH−]. For example, a solution with an H+ concentration of 10 ^−4 M will have a pH of 4, and a solution with an OH− concentration of 10^−9 M will have a pOH of 9. pH and pOH values can be estimated given a certain concentration using the following shortcut: p(N × 10−M) = (M−1).(10−N), such that a solution with an H+ concentration of 4 × 10−8 will have a pH = (8−1).(10−4) = 7.6.
Kw
pH and pOH are related to each other through the equation pH + pOH = pKw, where Kw is the autoionization constant of water (Kw = [H3O+][OH−] = 1 × 10−14 at 25°C). Thus, at 25°C, pH + pOH = 14. Thus, a solution with a low pH will automatically have a high pOH, and vice versa. pH is most commonly used, but it is important to be able to interconvert pH and pOH values if needed.
Acidic solutions have a high …………
Acidic solutions have a high H+ concentration and a low pH.
Basic solutions have a low…..
Basic solutions have a low H+ concentration and a high pH.
Bronsted Lowry Acid
proton donor
Bronsted lowry base
proton acceptor
lewis acid
electron pair acceptor
lewis base
electron pair donor
Take B, flip it get D, nice way to remember electron donor******
lewis acid/base
no H plus moving, definition more broad!!
strong acid
100% ionization, donate protons very easily** say process occurs 100% so 100% ionization, equilbirum so far to the right one arrow going right* everything turns into our products!
ex HCl
H2O + HCl –> H3O+ and Cl-
Ka= [H3O+] X [Cl-]/ [HCl] leave out water** is a pure liquid concentration doesnt change so LEAVE IT OUT of equilbirum expression**, super strong number in numerator and small number in denomatinor, so gives you a very big/high number of Ka, Ka much much greater than 1 here* so that is how we recognize a strong acid, an acid ionization constant much much greater than 1**