dental materials 93 Flashcards
force in
mg
m =
mass (kg)
g =
gravitational acceleration (10ms-2)
compressive strength
resistance to breaking from a force acting to reduce its size
tensile strength
resistance to breaking from a force acting to elongate
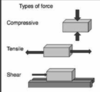
shear strength
resistance of a material to moving along an axis which is parallel to the forces direction
strain
change in length / original length
(L1 - L0) /L0
given as a ration or %

Young’s Modulus =
Stress /strain
F/A or (L1-L0)/L0
given in MPa
assess how rigid a material is

opposite of rigid is
flexible
fracture
large force causes a catastrophic destruction of materials structure
hardness
ability of surface to resist indenetation (KHN)
abrasion
material surface removal due to grinding
abrasion resistance
ability to withstand layers being removed compromising surface integrity
grinding along opposing tooth surface
fatigue
repititive ‘small’ stresses cause material fracture
creep
gradual dimensional change due to repetitive small forces (amalgam when it creeps above margins - standing proud then fracture)
deformation
an applied force may cause a permanent change in materials dimensions (not fracture it)
elasticity
impression materials - strain and recoverery
de-bond
applied forces sufficient to break material tooth bond by shear forces (ortho appliances)
impact
large sudden forces causes fracture - curve of upper dentures to accomodate palate maean that they are liable to snap
bonding to enamel
hetergenous structure (5% organic, 95% inorganic)
‘dry’
acid etch technique - remove cores of enamel prism leaving just peripheral enamel (creates pores for resin)
bonding to enamel is simple
bonding to dentine
dentine composition - 20% organic (collagen), 70% inorganic (hydroxyapatite), 10% water
fluid from pulp flows up dentine base making the surface wet
dentine varies - aged dentine more mineralised, pulpal dentine has increased moisture content
requirements of dentine bonding agent DBA
flowability
intimate contact with dentine surface
low viscosity
adhesion to substrate - mechanical, chemical, van der waals
smear layer is
pulp, dentine, bacterial debris plug dentine holes
what to do with smear layer
has to be removed by acid conditioning to either dissolve or solubilise the plugs
expose the tubules to create pores for resin
critical surface energy
the surface tension of a liquid that will just spread on the surface of a solid
importance of critical surface energy and dentine
a liquid must have a lower surface energy than the surface it is being placed on to flow and then stick
lower SE liquid will flow onto a higher SE substrate = lower SE as a whole
Wet Dentine has a lower SE than Composite filling materials
- Therefore this has to be reversed so that the Wet Dentine has a Higher SE than composite
- DBAs increase surface energy of dentine surface to allow composite to flow and stick
dentine adhesion through molecular entanglement
Adhesive absorbed onto surface but also into interior of dentine due to good wetting/surface energies
- absorbed component can polymerise
- polymer meshes with substrate- molecular entanglement = high bond strength
- phosphate-calcium bonds formed *can be hydrolysed by saliva/dentinal fluid = weakened bonds
3 components of total etch
dentine conditioner (acid e.g. phophoric 37%)
primer
bond
denitne conditioner in total etch
acid - phophoric 37%
- removes smear layer
- opens dentine tubules by removing smear plugs
- decalcifies upper layer of dentine
- etch washed off with water
- collagen network in this top 10μm
primer in total etch
*!Adhesive part of agent!*
- Hydrophilic end bond to dentine (think of philic and wetted dentine surface)
- Hydrophobic Methycrylate end bond to composite
Molecule has to have a spacer group to allow it to be flexible in bonding for all sites
Has a solvent (acetone, ethanol or water) to dissolve primer agent
bond in total etch
Resin that penetrates into dentine surface attaching to primers hydrophobic surface
- Mixture of resins (Usually Bis-GMA and HEMA)
- Predominantly Hydrophobic
- May contain filler and camphorquinone
- forms micromechanical bond within tubules and exposed dentine collagen- Hybrid layer (collagen and resin)
problems with total etch
- Overetching can cause collagen to collapse so no resin can penetrate
- Overetching can mean the depth of etch is too much for the resin to penetrate fully leaving areas of unsupported collagen
- Moisture dependence- too dry (dentine collapses) too wet (primer dilutes- reduced strength)
other option for DBA that isn’t total etch
Self Etching primer + Seperate adhesive
- DO NOT remove the smear layer- instead incorporate into bonding matrix
- not washed off!
- not as technique sensitive in terms of moisture but bond itsef not as good
Usually found as a one bottle solution (Self etch and adhesive)
uses of composite resin
primary caries
abrasion
erosion
failed restorations (secondary caries)
trauma
mechanical properties for composite resin
smooth surface finsih/polishable
technique sensitive
low setting shrinkage (bonding agents and good technique to maintain this)
thermal properties of composite resin
thermal expansion coefficient pretty poor compared to amalgam and GI
under cold stimulus the composite can shrink away from cavosurface margins
biological properties of composite resin
biocompatible - generally ok (unreacted monomer can be issue)
anticariogenic - gennerally not but some release F
5 classes of components for composite resin
filler particles
resin
camphorquinone
low weight dimethycrylate
silane (coupling agent)
types of filler particles in composite resin
conventional
- glass/quartz
microfilled
- microfine silica
hybrid
- combination of both
increased proportion of filler in composite resin =
decreased thermal expansion coefficient
role of filler in composite resin
- improve mechanical properties of material
- lower polymerisation contraction
- some fillers are radiopaque
- greater strength etc
resin in composite resin
BISGMA
- Bisphenol-A
- Glycidyle Methacrylate
- difunctional molecule - free radicals in teh resin facilitate C=C cross linking (free radical additon polymerisation)
role of camphorquinone in composite resin
initiator
blue light activation -> releases free radicals
- free radicals bond to BIS-GMA resin
confers increased molecular weight and so greater viscosity and strength
converts between 35-80% resin
- toxic unreactd monomer left potential
reacts with blue light at 44nm - depth of cure 2mm approx
role of low weight dimethycrylate in composite resin
TEGOMA - triethylene glycol dimethycrylate
inc proportion of TEGOMA = dec. viscosity
- almost like pain thinner
silane role in composite resin
coupling agent
acts as a wingman for glass to allow it to preferentially bond to resin and glass rather than water
- water would normally adhere to glass particles preventing resin bonding to glass
silane methoxy groups do the following:
- bind to absorbed water
- bind to OH groups in filler
what can be inc in self cure composite
benzoyl peroxide and aromatic tertiary amine
2 pastes, react together to break C=C bonds and release free radicals
what are crowns usually made of
procelain fused alloys
- porcelain on outside with a metal substructure
why use porcelain fused alloys
porcelain - good aeshtetic but microcracks form at the fitting surface = mechanincal failure
alloys - good mechanical properties
how can porcelain fused alloys restoration fail
fracture within porcelain itself
mechanical properities
compressive strength
stress needed to cause fracture
mechanical properities
elastic modulus (rigidity)
stress/strain ratio - i.e. stress needed to cause a change in shape
mechanical properities
brittleness/ductitility
dimensional change expereinced before fracture
mechanical properities
hardness
resistance of a surface to indent or abrasion
mechanical properties of porcelain
hard
strong
rigid
brittle (i.e. low tensile strength - can form defects, liable to fracture)
mechanical properties of alloy
hard
strong
rigid
ductile
porcelian metal resotrations properties
Metal Oxide sandwiched between porcelain and alloy
Metal Oxide also helps to eliminate cracks and defects on porcelain surface
Alloy acts as a support and limits the strain porcelain experiences
required properties for porcelian metal resotraions
- thermal expansion coefficient
- form good bond to porcelain
- avoid discoloration of porcelain
- mechnical
- melting
thermal expansion coefficient for porcelain-metal resotrations
its important that the alloy has a similar thermal expansion coefficient to the porcelain
REDUCES STRAIN
metal good bond to porcelain for porcelain-metal resotrations
will allow the restoration to have longevity and maximises supporting property of alloy
metal avoid discolouration of porcelain
for porcelain-metal resotrations
porcelain chose for aesthetics
silive in AgPd can produce a green discoluration
copper not used with High Gold due to discolouration
metal mechanical properties
for porcelain-metal resotrations
bond strength
- Gold (H/L), AgPd and CoCr all adequate (NiCr not)
hardness
- all adequate (NiCr too hard)
elastic modulus
- high (rigif) to support porcelain and prevent fracture
- NiCr best
melting
for porcelain-metal resotrations
recrystallisation temp of alloy
must be harder than fustion temp of porcelain or creep will occur
High Gold alloys
properties for porcelain fused restoration
- Match Thermal exp.
- Increased melting pt
- Forms oxide (Bonding)
- Biocompatible v good
- Cu presence can cause green discolourisation of porcelain
- Melting range too low
- Youngs modulus too low (Elastic)
low gold allous properties
for porcelain fused restoration
- Increased melting temperature
- Slightly better mech. props
- Biocompatible good
silver palladium alloys (AgPd) properties for porcelain fused restoraitn
High melting point
Care needed in casting
nickel chromium alloys (NiCr) properties for porcelain fused restoration
- High melting pt
- High YM
- Chromium forms oxide for bonding
- High casting shrinkage
- Not v biocompatible
- Lowish bond strength
cobalt chromium alloys properties for porcelian fused restoration
- High melting point
- Minimal casting shrinkage
- High YM
- High tensile strength
- High hardness
- Lowish bond strength
- Questionable Biocompatability
‘stressed skin’ effect
in porcelain fused alloys
Slight differences in thermal contraction coefficient
lead to compressive forces which aid bonding
chemical effects
in porcelain fused alloys
May be electron sharing in oxides
During firing porcelain flows and oxides in the metal oxide coating migrate
PMMA
polymethylmethacrylate
ideal properties of PMMA in general
- replaces function of natural teeth
- goes in pt mouth
- seen by others - aesthetics
- has to be cost effective
- dimensionally accurate and stable in use - fit and be retained
- high softening temp (Tg)
- must not distorrt when eating or cleaning
- unaffected by oral fluids over time
- non-toxic/non-irritant
- easy to repair
- radiopaque
- helps with detection of inhaled or ingested fragments if broken and swallowed
ideal properties of PMMA dimensionally
- dimensionally accurate and stable in use - fit and retained
- high softening temp (Tg) - must not distort when eating or cleaning
- unaffected by oral fluids over time
- high hardness and abrasion resistance
ideal mechanical properties of PMMA
- high YM
- high proportional limit - only large stresses will cause permanent deformation
- high transverse strength - upper denture has 3pt loading (2 lateral and 1 middle downward force)
- high fatigue strength - can withstand low stresses over a long time (design dependent)
- high impact strength - withstand large stresses applied rapidly e.g. dropping onto hard surface - may form hairline fractures

ideal thermal properties of PMMA
- artificial tooth - avoid internal stress on cool
- high thermal conductivity - so don’t burn throat due to not being able to sense hot liquids
ideal density for PMMA
low
aids retention - simple gravity law
setting reaction of PMMA
free radical addition polymerisation - adding two molecules of either same or different form to make a bigger molecule without elimination of smaller molecule (i.e. breaking C=C bonds)
4 stages in setting reaction of PMMA
activation
initiation
propagation
termination

activation in PMMA reaction
heat to 72oC or more releases radical molecules from symmetrical benzoyle peroxide molecule
initiaion in PMMA reaction
free radicals break down C=C bond in methacrylate monomer and transfer free radical
propagation in PMMA reaction
growing polymer chain
termination in PMMA reaction
of polymerisation
chain stops growing
2 components in heat cure acrylic
powder
liquid
powder constituents for heat cure acrylic
- initiator - Benzoyl Peroxide
- PMMA particles - pre-polymerised beads
- plasticiser - allows quicker dissolving in monomer liquid
- co-polymers - improve mechanical properties
liquid constituents for heat cure acrylic
- Methacrylate monomer - dissolves PMMA beads and polymerises
- Inhibitor - hydroquinone - prolongs shelf life by reacting with free radicals
- co-polymers - improve mechanical properites
why is it key PMMA has efficient polymerisation
increased molecular weight
=
better mechanical properties
undercured acrylic
- free monomer - irritant
- low molecular weight - poor mechanical properties
overcuring acrylic
- gaseous porisity
- voids in acrylic caused by monomer boiling
- polymerisation shrinkage
- monomer shrinks 20% due to poor packing, lack of excess material
- contraction porosity
crazing acrylic
fine cracks forming in material
metal is
aggregate of atoms in crystalline structure
alloy is
combination of metal atoms in a crystalline structure (metals are the building blocks of these)
FS
fracture strength

EL
maximum strength without plastic deformation

UTS
ultimate tensile strength

ductility
amount of plastic deformation prior to fracture

extent to which a material can be shaped/manipulated calculated by

(UTS/EL)%

stages of metal in stress strain diagram

upward curve - molten metal
flat line - liquid>solid
lower descending curve - cooling
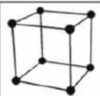
cubic
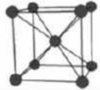
body centred cubic
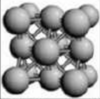
face centred cubic
crystal growth
atoms at these sites act as nuclei of crystallisation
crystals grow to form dendrites (3D branched lattic network)
crystals (or GRAINS) grow until they impinge on other crystals
region where grains make contact is called a GRAIN BOUNDARY

types of metallic grains
3
equi-axed grains
radial grains
fibrous grains
equi-axed grains
if crystals growth of equal dimension in each direction
radial grains
molten metal cooled quickly in cylindrical mould
fibrous grains
wire pulled through die
cold worked metal/alloy
3 ways to alter crystals
fast cooling
more nuclei, small fine grains
slow cooling
few nuclei, large coarse grains
nucleating agents
impurities or additives act as foci for crystal growth
grains are
each grain is a single crystal (lattice)
with atoms orientated in given directions (dendrites)
grain boundary
change in orientation of the crystal planes
(impurities concentrate here)
small fine grains area advantageous because (2)
but (1)
high elastic limit
increased UTS and hardness
decreased ductility
dislocation of columns (SLIP)
forces applied and defect moves along (propagation)
- when defect reaches grain boundary the lattic changes into new shape to free defect
dislocations - imperfections in crystal lattic
increases
- elastic limit
- UTS
- hardness
decreases
- ductility
- impact resistance

3 factors impeding dislocation movement
- grain boundaries (hence the fine grains)
- different alloys have different atom sizes
- when cold working builds up at grain boudaries

cold working
- down at low temp - below recrystallisation temperature so some changes can be made
- causes SLIP - dislocations collect at boundaries
- results in stronger harder material
- improves
- elastic limit
- UTS
- hardness
- decreases
- ductility
- corroision resistance
- impact resistance
RESULTS IN INTERNAL STRESSES
residual stress
not in perfect position - causes instability in the lattic
- results in distortion over time (undesirable!)
releived by annealing process
annealing
heating metal/alloy to cause thermal vibrations
- vibration cause migration of atoms
re-arrangement of atoms within grains
- doesn’t change mechanical properties or grain structure as a whole
care has to be taken as if temp too high causes grains to swell and poorer mechnical properties
recrystallisation
spoils cold work benefit but allows further cold working
continue bouts of cold work and recrystallisation until desired shape acheived
greater amount of cold work the lower recrystallisation temp
alloy
combination of 2 or more metals or metals with a metalloid (Si, C)
better mechanical properties than an individual metal
lower melting point than individual metal
definition of ‘phase’ used to define the metallic components of grains
physically distinct homogenous structure (can have more than one component)
defintion of ‘solution’ used to define the metallic components of grains
homogenous mixture at an atomic scale
one phase =
grains consising of metal A only
two phase =
individual grains of metal A+B in lattic network (distinct)
solution =
one phase but metal A+B in homogenous mixture (solid solution)
3 states upon crystallisation
be insoulble, no common lattic (2 phases)
intermetallic compound - with specific chemical formulation e.g. Ag3Sn
be soluble and form a solid solution (3 types)
3 stypes of solid solution
subsititution - atoms of one metal replace the other metal in the crystal lattic/grain
- random substition
- ordered substition
interstitial - atoms of markedly different in size - small atoms located in spaces in lattic/grain structure of a larger atom e.g. FeC
cooling curve - alloy
TL

crystallisation begins

cooling curve - alloy
TS

crystallisation ends

liquidus
temperature the alloy begins to crystallise

solidus
temperature where it solidifies (completely crystallised)

metals crystalise
at single temperature
alloys crystalise
over a temperature range

cooling and composition of alloys
must cool molten alloys slowly
allows metal atoms to diffuse through lattice
- ensure grain composition is homogenous
downside is it has hard grains - so poor mechanical properties
rapid cooling of alloys causes
coring

coring alloys
rapid cooling of alloys causes a concentration gradient to form
- different proportions of metal with one starting in low conc and increasing and the other metal antithesis of this
coring helps improve mechanical properties by reducing dislocation movement and resulting in small grains

homongenising anneal
gentle heat and vibrate atoms, helps atoms to diffuse (below recrystalistallisation temperature) to reduce the coring but not altering the grain structure allowing a more homogenous proportion of metal right the way through its depth

dislocation in alloys
in metals the defect rolls smoothly over the lattic along the slip plane
in alloys the defect has to ‘fall’ into the space between the large and small atoms and climbs over the atoms alonge the grain boundary but requires a great degree of effort to do so
- instead of flying over a crowd of people you have to climb over them because you cant get through them*
- Requires more stress to move dislocations in a solid solution = inherently better mechanical properties
eutetic alloys
alloy melts at a temperature higher than that of its individual metals (together = stronger)
eutetic alloys properties
physically distinc grains
soluble in liquid state
insoluble in solid state
unusual in that it is an alloy which cools at a single temperature not over a range
function of impression material
produce an accurate replica of the surface and shape of hard and soft oral tissues
impression definition
negative reproduction of tissues
mucostatic
e.g. ZOE, low viscosity alginates
fluid materials that displace the soft tissues slightly - i.e. give an impression of the undisplaced mucosa
mucocompressive
e.g. impresion compound, high viscosity alginates/elastomers
viscous materials that record an impression of the mucosa under load i.e. give impression of displaced soft tissue
viscoelastic behaviour
it is advantageous to wait for a time TF - TL after removing the L tray before you pour the cast - so as to minimise permanent strain (deformation)
remove the tray with a sharp pull to minimise permanent deformation
3 elastic impression materials
(not perfectly elastic!)

hydrocolloid - alginate
elastomers
hydrocollid
A colloid is a 2 phase system of fine particles
(1-200nm) of one phase dispersed in another phase (water is dispersing medium in hydrocolloid)
e. g. irreversible alginate
* reversible agar (no longer used as cross infection)*
alginate reaction
irreversible hydrocolloid
2 NanAlg + nCaSO4 ——> nNa2SO4 + CanAlg
Cross linking with Calcium allows the alginate to set
- intermediate reaction between sodium to calcium stage allows a delay in setting
- Use perforated tray with adhesive for alginate!
- remove tray with a sharp pull
- large bulk reduces strain on material

alginate composition
Sodium alginate (reacts w/ calcium ions)
Calcium Sulphate (provides calcium ions)
Trisodium sulphate (delays gel formation)
Filler (increases cohesion and strength)
Modifiers, flavourings, chemical indicators (surface, taste, pH)

2 elastomers
polysulphides
polyethers (impregum)
silicone
elastomers properties
monophase impression material
can break stock trays - must make special tray
made of prepolymer, catalyst and filler
undergoes addition polymerisation type reaction
2 non elastic impression materials
impression compound
impression paste
imp material accuracy needs
low viscosit
wetting ability
impression material dimensional stability needs
immediate - on setting (contraction), on removal (deformation, viscoelasticity, rigidity)
long term
impression material handling characteristics needs
strength
tear resistance
needs for imp materials
accuracy
dimensional stability
handling characteristcs
cost
taste
colour
decontamination policy for imps
wash under running water
soak in perform for min 10 mins
wash under running water again
bag in moist tissue
perform is
potassium perozomonsulphate/sodium benzoate
stock imp trays
need to be rigid
esp for single stage imp techniques involving putties

custom imp trays
acrylic - cold cure or light cure
chairside silicone
can be modified with green stick

perforations in imp trays
allows material to latch onto tray to prevent separation occuring (alginate)
non perforated trays used with compound

cross section of imp trays
helps direct impression material
square - dentate pt
oval/rounded - edentulous

components of stainless steel
72% iron
19% chromium
8% nickel
- 7% Titanium
- 3% carbon
stainless steel % iron
72%
stainless steel % chromium
18%
stainless steel % nickel
8%
stainless steel % titanium
1.7%
stainless steel % carbon
0.3%
iron role in stainless steel
main constituent and when combined with carbon forms steel
chromium role in stainless steel
lowers the temperature at which martensitic SS forms i.e. main component of hard SS used in ortho
nickel role in stainless steel
lowers critical temp that the austentite breaks down on cooling
improve the corrosion resistance of the alloy
improves strength
titanium role in stainless steel
prevents precipitation of chromium carbides at the grain boundaries
iron
solid state phases
allotropic - undergoes 2 solid state phase changes
1) Temp >1400oC - Body centred cubic lattice with low carbon solubility (0.05%)
2) Temp > 900-1400oC - Face centred cubic lattice with higher carbon solubility (2%)
3) Temp > <900oC - Back to form in stage 1with temperature

solid solutions iron forms with carbon
Austenite
- Interstitial solid solution
- face centred cubic which exists at >720oC
Ferrite
- Very dilute solid solution, exists at low temp
Cementite
- Fe3C, exists at low temp
Pearlite
- Eutectoid (minimum transformation temp between solid solution and simple mixture)
- mix of ferrite and cementite (i.e because they are both at low temp)

aistentite quenched –>
martensite
martenstie
formed because there is no time for diffusion of carbon through the lattice
has a distorted BCC lattice
is very brittle but this can be lessened by tempering
tempering of iron
Heating at 450oC following quenching, helps to control poor mechanical properties
how much chromium if stainless steel
>13% chromium
chromium provides
corrosion resistance due to chromoium oxide layer in SS
but can be attacked by chlorides
Lowers the Austenite to Martensite temperature
Lowers the Austenite to Martensite transition rate
Decreases the % of carbon which forms a Eutectoid
martensititc SS
12-13% Chromium + little carbon
Heat hardenable (tempering process)
dental instruments often mate of
autenititic SS
contains sufficient Chromium and Nickel to suppress austenite to martensite transition i.e more than normal amounts of these metals
Used in
- sterilisable instruments which don’t have a cutting edge
- Ortho wire, due to them being readily cold worked and their corrosion resistance
- Sheet form for denture bases
Corrosion resistance is more important than strength and hardness
wrought alloys definition
manipulated by cold working
i. e. are drawn into wire shape
e. g. ortho wire and partial denture clasps
requirement of wires
5
- high springiness/ Elastic Modulus (YM)
- stiffness (High Young’s Modulus)
- high ductility
- easily joined without impairing properties i.e. soldered or welded
- corrosion resistant
springiness
ability of a material to undergo large deflection (to form arc) without permanent deformation i.e. returns to its original shape
weld decay
occurs at 500o-900oC
chromium carbides build up at grain boundaries
alloy becomes brittle
- less chromium in central region of solid solution
- periphery more susceptible to corrosion
solution -> low carbon steel, stabilised stainless stel with small amounts of titanium which forms carbides preferentially and not at grain boundaries
dentura base made of stainless steel
advantages
- much thinner than acrylic
- lighter
- fracture resistant
- corrosion resistant
- polishable
- high thermal conductivity
- high impact strength - won’t fracture when dropped
- high abrasion resistance
denture base made of stainless steel
negatives
- dimensional inaccuracy during die process
- elastic recovery of steel can lead to inaccuracy
- damage of die under hydraulic pressure
- loss of fine detail
- difficult to ensure uniform thickness
3 stages of dental ceramics setting reaction
vitreous phase
fusion
firing (sintering)
virteous phase of dental ceramics setting reaction
formed by a flux i.e. Feldspar, this breaks the terahedral structure of silica
forms an amorphous 2D structure
feldspar lowers the fusion and softening temperature of the glass
during firing it forms a solid mass around the other components
fusion phase of dental ceramics setting reaction
feldspar reacts with the outer layers of silica known as Kaolin - melding the particles together and forms leucite at 1150-1500oC
molten mass then is quenched and ground to a fine powder known as frit
this stage that you incorporate opacifiers, metal oxides and crystalline alumina for colouring and strengthening
firing phase of dental ceramics setting reaction
(sintering)
heating leads to sintering
occurs just above the glass transistion temperature
glass phase will soften and the particles coalesce
causes contraction of the material by about 20%
composition of dental ceramics
4 elements
feldspar
silica
kaolin
metallic oxides
feldspar in dental ceramics
potassium aluminium silicate and sodium aluminium silicate
potash - PAS
soda - SAS
silica in dental ceramics
formed by tetrahedra
when reacting it forms an amorphous high melting point glass which bonds to the feldspar
Kaolin in dental ceramics
china clay
confers opacity on porcelain and contributes to the formation of the glass matrix
becomes sticky when mixed which allows the porcelain to be worked to shape a crown
metallic oxides in dental ceramics
small amounts of metallic oxides provided
blended with unpigmented frit
properties of dental ceramics
5
aesthetics
chemical stability
thermal properties
dimensional stability
mechanical properties
aesthetics of dental ceramics
colour stable
retain surface very well without staining
smooth surface and have great optical properties
chemical stablity of dental ceramics
very stable
unaffected by the pH changes and ranges in the mouth
doesn’t stain
good biocompatability
thermal properties
similar to tooth subtance
similar thermal expansion coefficient to dentine
thermal diffusivity low too
dimensional stablity of dental ceramics
20% shrinkage when fired but in the mouth literally no change
mechanical properties of dental ceramic
very hard
can damage opposing teeth if not glazed
high compressive strength
very low tensile strength
3 problems with dental ceramics
static fatigue
surface micro-cracks
slow crack growth
together mean feldspathic ceramics can only be used in anterior region
static fatigue in dental ceramics
decrease in strength over time in the absence of any applied load, thought to be due to hydrolysis of Si-O groups within the material over time in an aqueous ennviroment
surface micro-cracks in dental ceramics
can occur during manufacture, finsihing or due to occlusal wear
slow crack growth in dental ceramics
cyclic fatigue under occlusal forces in a wet environment over time
3 types of copings
metal copings (porcelain fused to metal alloy)
alumina core
zirconia core
alumina core
core material in Porcelain Jacket Crowns
better flexure strength than feldspathic porcelain
alumina particles stock cracks propagating
however its opaque so can’t be used as a restorative material
zirconia core
zirconium dioxide
- zirconia powder doesn’t sinter unless heaated at over 1600oC
- pure zirconia can crack on cooling
- zirconia is a monoclinical structure
- Ytrria stabilisation - allows zirconia to be used as a bridge
- zirconia goes through manufacturing process and is veneered with feldspathic porcelin to produce the final restoration
Yttria stablisation of zirconia
zirconia contains small amounts of Yttria
Yttria has a tetragonal structure (zirconia is monoclinical)
crack starts, tip of crack causes the yttria to reach a critical stress level and causes it to convert to the monoclinical structure like the rest of zirconia which will manifest as the crack is healing over
allows it be used for bridges
pros of zirconia core
great aesthetics because of cores opacity
excellent fit
cons of zirconia core
expensive equipment initial but material is cheap
porcelain can debond from core
inert fitting surface so cannot etch or bond
cast/pressed ceramics process
lithium disilicate/reinforced glass is the ceramic used in this
wax up restoration
invested
cast from ceramic ingot
undergoes ceraming i.e. reheating it once cast to improve crystal structure and produce crack inhibiting crystals
ceraming stages (2)
1 - crystals formed: max no. nuclei formed
2 - crystal growth: max physical properties
luting crowns
any silica containing crowns can be etched with hydrofluric acid to produce a retentive surface
- this surface can be bonded to using a silane coupling agent and then bonded to the tooth using an appropriate agent
Zirconia crowns are inert so cannot be ethced, howevere strong enough to be self supporting and can be bonded with a traditional cement
milled Vs cast/pressed crowns
milled crowns have consistent physical properties meaning they are better overall
key properties of luting agents
- viscosity/film thickness
- ease of use
- radiopaque
- marginal seal
- biocompatibility
- mechanical properties
viscosity film thickness of luting agent
dependent on size of powder/filling particles
must be low to allow seating of restoration without interference
viscosity increases as the material sets - highlighting the importance of seasting it quickly and with pressure
25 micro-metres thickness ideally
ease of use for luting agents
easy to mix
working time should allow seating of restoration
should have short setting time
radiopaque quality of luting cement
some ceramic crowns are radiolucent
radiopacity allows marginal breakdown to be illicited on radiographs
marginal seal of luting cements
ideally should bond chemically to the tooth and form an inpenetrable bond
biocompatablity of luting agents
non toxic
low thermal conductivity
pulp friendly
mechanical properties of luting agents
high compressive strength
high tensile strength
high hardness
YM similar to tooth
powder components of zinc phosphate cement
zinc oxide - main reactive ingredient
magnium dioxide - gives white colour and compressive strength
other oxides (alumina, silica) - improve physical properties and alter shade of material
liquid components of zinc phophate cement
phosphoric acid - aq solution
aluminum oxide - ensures consistency of set material
zinc oxide - retardant to reaction giving bettern working time
setting reaction of zinc phosphate cement
acid/base
- ZnO + 2H3PO4 -> Zn(H2PO4) + H2O
hydration reaction
- ZnO + Zn(H2PO4) + 2H2O -> Zn3(H2PO4)2 . 4H2O
- makes Hopiete
aluminium oxide role in setting reactin of zinc phosphate cement
prevents crystallisation leading to an amorphous glassy material
glassy matrix of acid salt surrounding unreacted ZnO powder
Matrix is almost insoluble but is porous and contains free water from the setting reaction
the cement matures and binds to the water leading to a stronger less porous material
evaluation of ZnO
Low initial pH of 2 can cause pulpal irritation
Exothermic setting reaction
Not adhesive to tooth or restoration - acts almost like grout - just filling in spaces
Not cariostatic
final set takes 24 hrs
brittle
opaque
evaluation of zinc polycarboxylate cement
Similar material to Zinc Phosphate cement but instead of Phosphoric acid it is replaced with Polyacrylic acid
Bonds to tooth surfaces a bit like GIC
Less exothermic
pH is low to begin with but returns to normal faster, long chain acids less damaging to the dentine
cheap
but
- difficult to mix
- difficult to manipulate
- soluble in oral environment at low pH
- opaque
- lower modulus and compressive strength
evaluation of glass ionomer cement
- low shrinkage/stablity
- relativly insoluble once fully set
- aesthetically better than ZnPhos
- self adhesive to tooth substance
- F release
- cheap
- highly soluble
composite luting cement
bonding to indirect composite
- composite bonds to composite
- micromechanical bonding occurs on the rought internal surface of the composite inlay
- bond is also chemical to remaining unbroken C=C bonds on the inlay surface
- using a dual curing cement as light penetration using conventional cement wouldn’t work
composite luting cement
bonding to porcelain
is brittle and is required to be bonded to tooth to prevent fracture
untreated porcelain is smooth and non-retentive
can be treated with HF to etch the surface (v.toxic)
produces a rough retentive surface but is still not hydrophobic and compatible with composite resin luting agents
requires a surface wetting agent - silane coupling agent
thin porcelain - light cure composite can be used
thick porcelain - dual cure needed
silane coupling agent when composite luting agent is bonding to porcelain
allows a strong bond to form between the silcon group in the porcelain and the base carbon as part of the composite monomer
composite luting agent bonding to metal
like porcelain, composite doesn’t directly bond to metal
metal surface needs roughened - etching or sandblasting
electrolytic etching (beryllium alloys best)
need a dual cure luting agent
composite luting agent bonding to non precious metals
use carboxylic/phosphoric acid derived materials
MDP and 4-META
both molecules have an acidic C=C end, this reacts with the metal oxide and renders the surface hydrophobic
bonding to precious metals with composite luting agent
change to precious allow composition to allow oxide formation
increase copper content and head to 400oC
sulphur based bonding agent
self etching composite resin luting agents
acid groups bind with calcium in HPA forming a stabilising attachement between tooth and resin
ions from dissolution of filler neutralise the remaining acidic groups forming a chelate reinforced methacrtylate network
limited removal of smear layer or significant infiltration into the tooth suface (only a couple of microns)
good bond strength to dentine
self etching composite resin luting agents
bond to enamel vs dentine vs ceramics vs metals
enamel
- lower than dentine
- should be etched with acid prior to application
dentine
- better than to enamel
- no need to etch
ceramics
- brand specific - RelyX unicem bonds quite weel to sandblasted Zirconia
metal
- better to non-precious metal
- not good enough for ortho brackets
temporary cements
dont fully set and remain soft so can be removed easily
prep must be physical
base
- ZnO
- start
- mineral oil
accelerator
- resins
- eugenol or ortho EBA
- carnuaba wax
wax weakens the structure of the set cement and makes it easier to remove
material can be modified to make it weaker still by incorporating petroleum jelly into the mixture
eugenol not used when permenanet cement willl be resin as it inhibits set
addition cured silicones
polyvinylsiloxanes
elastomer imp material
N.B Contain PDS but some of the methyl groups replaced by Hydrogen and Vinyl hence termed PS instead of PDS
Base paste
- polydimethylsiloxane - some methyl (CH3) groups replaced by hydrogen
- filler - variations change viscosity
Catalyst paste
- polydimethylsiloxane - some methyl groups replaced by vinyl (CH2 =CH)
- filler - variations change viscosity
- platinum catalyst eg chloroplatinic acid
Base Paste ((PDS (Hydrogen)) + Catalyst ((PDS) Vinyl)) + Chlorplatinic acid ——> Cross linked Polymer formed
(NO BYPRODUCTS)
Hydrophilic Silicones
- incorporate non-ionic surfactant
- wets tooth surface
- more easily wetted by water containing die materials
condensation cured silicones
polydimethyl siloxane
elastomer imp material
TYPE 1 reaction
- Silicone Polymer + Organhydrogen Siloxane (cross linking agent) ——> Cross linked Silicone Polymer + Hydrogen Evolved
TYPE 2 reaction
- Silicone Polymer (Double OH) + Alkoxy orthosilicate (cross linking agent) + Silicone Polymer (Single OH) —-> Cross Linked polymer + Alcohol
- within the monomer rather than the ends of chains
polyether components
base paste
- amine terminated prepolymer for cross linking
- inert filler (viscosity and strength)
catalyst
- ester derivative of aromatic sulphonic acid initiates polymerisation
- inert oils and filler form paste
polyether + sulphonate ester -> cross linked material
3 phases of polyehter and sulphonate ester setting reaction
1) Activation > Sulphonate Ester ionises and provides cations (+) to the reaction
2) Initiation > Cations then open up Epimime rings in the prepolymer which releases a further cation
3) Propagation > The chain reaction of cation release continues and the now ionic prepolymers join to form a larger chain as the ions are passed along
3 key factors for analysing imp materials
viscosity
surface wetting
contact angle
viscosity of imp material
determines a materials potential for making close contact with soft tissue surfaces = recording surface detail
surface wetting for imp material
must make intimate contact with teeth/mucosa

contact angle for imp material
determines how well the material envelopes hard/soft tissue surfaces
accuracy considerations for imp materials
reproduction of surface detail - should reproduce at least 50μm of detail
viscoelasticity/elastic recovery- withdrawing impression quickly, less permanent deformation from strain

dealing with removal from undercuts for imp materials
assessment
flow under pressure - shark fin test
tear tensile strength on removal
rigidity on removal
dimensional stablity for imp materials assessment
should be low setting shrinkage
thermal expansion/contraction should be low - due to the disparity in temp from the oral cavity to the outside environment
storage - some materials undergo synersis or imbibition causing dimensional change
stress concentration
abrupt changes in shape of a file that leads to a higher stress at that point aka a notch in the file
shape memory
when elastic limit is significantly higher than that of conventional metals, will deform when heated returb to its original shape
plastic deformnation
permanent bond displacement occuring when elastic limit is exceeded
plastic limit
the point in which a plastically defomed file breaks
cyclic fatigue
freely rotating in a curvature
generaton of tension and compression cycles
cyclic fatigue
failure
torsional failure
when the load is suddently revered
i.e. turning the file in the opposite direction to which has been
mechnical agitation
moving a substance by external vibration to allow said substance to fill a space
preparation of the canal for obturation
smear layer formed during preparation
- organic pulpal material and inorganic dentinal debris
- superifical 1-5μm with packing into tubules
- bacterial contamination, substrate and interferes with disinfection
- also prevents sealer penetration
removal of smear layer
- 17% EDTA
- 10% citric acid
- MTAD (mixture of a tetracylic isomeer, an acid and detergent)
- sonic and ultrasonic irrigation
- watch apical control
EDTA and NaOCl in canal
should never be in the canal at the same time
because the EDTA neutralises the effectiveness of the NaOCl
also forms papachloanailine which is cytotoxic and carcinogenic
ideal properties of obturation materials
- easily manipulated with ample working time
- seals the canal laterally and apically
- non-irritant
- impervious to moisture
- unaffected by tissue fluids
- inhibits bacterial growth
- radiopaque
- does not discolour tooth
- sterile
- easily removed if necessary
gutta percha is
natural rubber and GP are polymers of the same monomer - transpolyisoprene
comes in 2 forms:
- α - used in themoplastic manipulation techniques, the natural occuring form and when heated above 65o becomes amorphous
- β - more commonly used in cold lateral compaction - rapidly cooled and recrystalised alpha, used in commercially produced GP
GP formulations are 60-75% Zinc Oxide
inc a variety of other agents to allow them to be radiopaque on radiographs
ideal properties of sealing material
- exhibit tackiness to provide good adhesion
- establishes a hermitic seal
- radiopacity
- easily mixed
- no shrinkage on setting
- non-staining
- bacteriostatic or does not encourafe growth
- slow set
- insoluble in tissue fluids
- soluble on retreatment
types of sealers
- ZOE
- GI sealers
- resin sealers
- calcium silicate sealers
- medicated sealers
ZOE sealer
- effective antimicrobial
- offers cytoprotection
- resin acids are 90% of the colophony (Rosin) in the material and these are strongly antimicrobial and cytotoxic
- altough toxic may be overall beneficial with longlasting antimicrobial effect and cytoprotection
- formation of eugenolate consititutes hardening, this is accelerated by CaOH so this must be removed from the canals
- remaining eugenol can act as an irritant
- lose volume with time due to dissolution - resins can modify this
GI sealers
- advocated due to dentine bonding properties
- removal upon retreatment is difficult
- minimal antimicrobial activity
- not enough data yet
3 types of resin sealer
AH plus
Epiphany
EndoRez
AH plus resin sealer
- long history of use - development of AH26
- epoxy resin
- paste-paste mixing
- slow setting - 8 hrs
- good sealing ability
- good flow
- initial toxicity declining after 24hrs
epiphany
resin sealer
dual cure dental resin composite sealer - used with Resilon
- BisGMA
- Ethoxylated BisGMA
- Urethane-dimethacrylate UDMA
- hydrophillic difucntional methacrylates
- fillers of calcium hydroxide, barium sulphate, barium glass and silica
requires self-etch primer
EndoRez
UDMA resin-based sealer
- hydrophillic
- good penetration into tubules
- biocompatible
- good radio-opacity
Calcium silicate sealers
- high pH (12.8) during intial 24hrs of the setting
- hydrophillic
- enhanced biocompatibility
- does not shrink on setting
- non-resobable
- excellent sealing ability
- quick set - 3-4hrs - requires moisture
- easy to use
medicated sealers
sealers containing paraformaldehyde not acceptable
lead and mercury components removed
severe adn permanent toxic effects on periradicular tissues
sargenti paste, endomethasone, SPAD
how are investment materials used
wax pattern made (e.g. crown, inlay etc)
investment material poured around wax pattern and allowed to set (mould)
wax then eliminated (boiled out)
molten alloy is then forced into the cavity left by the wax via sprues prepared in the investment material
components of all investment materials
refractory - silica (quartz/tridymite/crisotbalite)
binder - gypsum/phosphate/silicate
modifiers - change physical properties
gypsum bonded material
Supplied as powders and mixed with water and silica and calcium sulphate hemihydrate with other components that control setting time
- quartz withstands high temps and gives expansion
Thermal inversion is when the silica is heated so that it undergoes a phase transformation and expands (alpha > beta quartz)
Above 320oC there is contraction of the investment material which causes water loss, this can be reduced by modifiers like sodium chloride and boric acid
Heat Soaking
- > 700oC
- CaSO4 + 4C ——> CaS + 4CO
- (THEN) 3CaSO4 + CaS ——> 4CaO + 4SO2
- Allow heat soaking to complete to allow gases to escape
Chemical stability
- <1200oC satisfies requirements
- >1200oC problems with sulphur trioxide production - causes corrosion and porosity in alloy castings
- Therefore only allots with melting point of <1200 allowed
phosphate bonded materials
Powder
- Silica
- Magnesium Oxide
- Ammonium Phosphate
Liquid
- Water
- Colloidal Silica (increases strength, undergoes hygroscopic expansion)
Magnesium Oxide + Ammonium Phosphate + Water/Colloidal Silica —— > Magnesium Ammonium Sulphate
Type 1 PBMs
- Inlays, crowns and other fixed restorations
Type 2 PBMs
- RPDs and other cast removable restorations
- Don’t require outer casting ring
silica bonded materials
- Powdered Quartz/Cristobalite bonded with silica gel
- Silica gel becomes Silica and is a tightly packed mass of particles
- Binder is usually Ethyl Silicate with Hydrochloric acid and Industrial spirit
- the ethyl silicate is hydrolysed releasing alcohol and forming silica gel
- the Hydrolysis and gelation can be accelerated by Piperidine but this causes alot of shrinkage
- Ethyl silicate mediated materials dont dimensionally change on setting because they are Thermal expanders
- their linear expansion = their linear thermal expansion unlike gypsum/phosphate
GIC polyacid components
- polyacrylic acid (ionic monomers)
- copolymers of acrylic and itaconic acid
- or Acrylic and maleic acid
- tartaric acid
- added to control the setting characteristics of material
powder (base, metal) components of GIC
- silica
- alumina
- calcium fluoride
- aluminium fluoride
- alumonium phosphate
- sodium fluoride
adding Strontium + lithium salts increase radiopacity but dont take part in reaction
setting reaction of GIC phases
- dissoluion
- gelation
- hardening
reaction for GIC
MO.SiO2 + H2A -> MA + SiO2 + H2O
M=metal A=polyacid
glass + acid -> salt + silica gel
dissolution phase for GIC
Acid into Solution
H+ ions attack the glass surface
Ca, Al, Na and F ions are released
Leaves silica gel around the unreacted glass
gelation phase for GIC
Inital set due to Ca2+ ions cross linkinh w/ polyacid by chelation with carboxyl groups
Bivalent so can bind to two carboxyl groups
Chelation can happen twice on same molecule of polyacid (gels toonmuch?)
At this point the material will appear hard in the mouth (after a fewminutes) caused by formation of calcium polyacrylate
hardening phase for GIC
Trivalent Aluminium increases crosslinking
Formation of Aluminium Polyacrylate
Helps mechanical properties greatly
imp points for GIC setting
Must be moisture and dessication free i.e not too wet or dry
This is most important during the hardening phase
if not achieved aluminium will leach out- less cross linking, water lost from matrix if dessicated, saliva contamination causes excess water absorption = A WEAK MATERIAL
protection for conventional GIC
- Varnishes
- Copal ether
- Acetate
- Resins
- Dentine/Enamel bonding agents
- Unfilled Bis-GMA resins
- Greases/Gels
- Vaseline (not great as removed quickly by lips and tongue)
properties of GIC
Can bond to enamel and Dentine w/o use of intermediate material
Bond strength pretty poor (5-20 MPa)
Poor Tensile strength
Lower compressive strength than composite (less than 50%)
Higher solubility than composite due to unprotected material during gelation phase
Usually seals well
Fluoride release (for short time)
Bonding**
- Carboxyl (COO groups) in cement bond to Ca in the enamel!
- In addition there is Hydrogen bonding and metallic ion bridging to the collagen in enamel too
- IT IS CONDITIONED NOT ETCHED!! W/ POLYACRYLIC ACID!!
powder components of RMGIC
- Fluro-Alumino-Silicate glass
- Barium glass (provides radiopacity)
- Vacuum dried polyacrylic acid
- Pottasium persulphate (redox catalyst - cures resin in the dark)
- Ascorbic acid
- Pigments (vary in shade for aesthetics)
liquid components of RMGIC
- HEMA (water miscible resin)
- Polyacrylic acid with pendant methyacrylate (undergo acid/base reactions and polymerisation)
- Tartaric Acid (speeds up setting reaction)
- Water (allows reaction between polyacid and glass)
- Photoinitiators - ALLOW LIGHT CURE
two types of setting reaction for RMGIC
dual curing
tri curing
dual curing RMGIC
On mixing proceeds like the normal GIC (dissolution)
Light activation causes free radical methycrylate reaction to occur = resin matrix formed
Acid Base reaction occurs for several hours afer
tri curing for RMGIC
On mixing proceeds like the normal GIC (dissolution)
Redox reaction begins
Light activation- resin matrix formed
Redox reaction continues for 5 mins after initial mix
Acid base reaction occurs for several hours
Final hardening may take days
RMGI vs GI
Better physical properties
Lower solubility
Fluoride release
Better translucency/aesthetics
Better handling
role of cavity liners
- prevents gaps
- acts as a protective barrier
e.g in Amalgam
Cavity base lining placed in bulk to block undercuts for metal restorations
lining for exposed dentine- promotes pulpal health
liner as a pulp protection
protects from
- chemical stimuli - unreacted chemicals in filling material/pH of filling material
- thermal stimuli - exothermic setting of composite#heat conduction amalgam/gold
- bacteria + endotoxins - microleakage (against oral fluids and bacteria and their toxins ingressing between material and cavosurface margins)
liner therapeuatic and palliative role
therapeutic role in calming down pulpal inflammation and promote healing
palliative reduce symptoms in pts with irreversible pulpitis
ease of use for liners
should be command set
workable
easy to mix
thermal properties
conductivity
how well heat energy is transferred through a material
Heat flow through a cylinder of unit cross sectional area with a temp difference of 1oC between both ends
Cavity lining should have as low thermal conductivity as possible!!
thermal properties
thermal expansion coefficient
change in length per unit length for a rise of 1*C (in ppm*C-1)
Liner should match thermal coefficient of tooth!
GIC has better TEC than RMGI
thermal diffusivity
similar to conductivity
measured in cm2/sec
Liners have similar or lower thermal diffusivity than enamel
Amalgam much higher than tooth tissue- hence use of liner
desired mechanical properties for cavity liners
High compressive strength
allows placement of filling without breakage
similar modulus to dentine
Radiopaque
Marginal seal
low solubulity
Cariostatic (fluoride release/ antibacterial)
Biocompatability (non toxic, pH neutral, not exothermic)
4 types of cavity liners
calcium hydroxide liner (dycal, life)
zinc oxide cements
resin modified zoe
GIC/RMGIC
calcium hydroxide liner
dycal, life
Components
- Base- Calcium Hydroxide, Zinc Oxide Filler, Plasticiser
- Catalyst- Butylene glycol Disalicylate (reactive element), filler, radiopaquer
Setting reaction
- Butylene glycol disalicylate + Zinc Oxide filler = chelation and at pH 12
Mode of action
- irritates odontoblast layer- forms reparative dentine, calcium from pulp helps to form a bridge between pulp and dentine, high alkaline content is bactericidal
zinc oxide cements used as cavity base
Zinc Oxide Eugenol- used as a cavity liner, PD dressing and root canal sealer
Eugenol can have obtundant effect (reduce dentine sensation) and reduce pulpal pain
*inhibits set of composite resin material!! shouldn’t be used!
Zinc Oxide + Eugenol= Zinc Oxide Eugenolate + Water
(Base) + (Acid) = (Salt ) + (Water)
resin modified ZOE as cavity base
adds resin to the ZnO/Eugenolate matrix to reduce solubility
GIC/RMGIC as base or liner
can actually bond Amalgam to tooth as the bond goes Tooth—RMGI—Amalgam (it bond to rest.material)
Palliative cements- Base (seldom used)
powder components of amalgam
50% by weight - mainly silver, tin and copper
- Silver and Tin- intermetallic compound Ag3Sn
- Copper- increases strength & hardness
- Zn - scavenger during production - preferentially oxidises & slag formed / removed - some zinc free
- *Hg in powder - (few materials)– “pre-amalgamated” alloys - react faster
liquid amalgam components
- Hg (50% by weight)
triple distilled (very pure) – reacts with other metals
particle types for amalgam
lathe cut (coarse medium or fine) - formed by filing ingots
spherical/spheroidal - range of particle sizes, formed by sparying molten metal into inert atmosphere
setting reaction for amalgam
Silver/Tin Powder reacts with Liquid Mercury
Some unreacted Silver/Tin Powder remains
Silver Mercury (y1) and Tin Mercury (y2) form amalgam matrix
Modern amalgam sets with a small amount of contraction (due to a solidsolution of mercury forming in with the Silver/Tin (only -0.2% contraction so very small)

effect of Zn in amalgam clinically
Zn + H20 (saliva) -> ZnO + H2 (gas)
Hydrogen gas causes pressure expansion of amalgam
downward pressure = pulpal pain
upward pressure= sitting proud of surface - chipped off
∴ Zinc free materials!

factors affecting Amalgam strength
Amalgam strength usually ok after 24 hrs however is decreased by the following
- Undermixing
- Too high Hg content after condensation
- poor condensation pressure
- slow rate of packing- increments dont bond
- corrosion by oral fluid
corrosion of amalgam
y2 most electronegative (tin and mercury)
this weakens amalgam particularly at the margins
orrosion products may actually hep with sealing margins
reduce corrosion by
- copper enriched materials
- polishing margins
- avoiding galvanic cells (stops random redox reactions from occuring due to two different metals with different electronegavity contacting)
Cu enriched amalgams
- Gives higher early strength
- Less creep
- Higher corrosion resistance
- Increased durability of margins
either
- single composition formulations
- dispersion modified setting reaction



