Colon Cancer Flashcards
What type of cancers make up 95% of colorectal cancer cases?
Adenocarcinomas
- Neoplasia of epithelial tissue that begins in gland forming cells that produce mucus
- The colon has numerous glands within the tissue
- When these glands undergo genetic change they move from benign to invasive, malignant colon cancer
- Colon cells lose the APC (Adenomatous Polyposis Coli ) tumour suppressor gene and become a small polyp
What is the most common gene mutation in colorectal cancer?
APC
(Adenomatous polyposis coli)
- The APC protein is a negative regulator that controls β-catenin concentrations and interacts with E-cadherin (involved in cell adhesion).
- For cancer to develop, both alleles of APC must be mutated.
- FAP - caused by inherited, inactivating mutation of APC gene, most of these mutations cause a truncated and nonfunctional APC.
- This shortened protein cannot suppress the cellular overgrowth that leads to the formation of polyps, which can become cancerous.
What condition is caused by an inherited, inactivating mutation in the APC gene?
Familial adenomatous polyposis (FAP)
- Inherited condition in which many adenomatous polyps form in the epithelium of the large intestine.
- These polyps begin as benign but undergo malignant transformation into colon cancer.
- A flawed APC gene inhibits the tumour suppressor properties of APC.
How many deaths does colorectal cancer cause per year in the UK?
15-20,000 deaths/year in the UK
What are the main roles of the small intestine?
Small Intestine
- Majority of digestion and absorption of food occurs.
- Three distinct regions: duodenum, jejunum and ileum.
- Villi project out into the lumen of the gut to maximise nutrient absorption.
What are the main roles of the large intestine?
Large Intestine
- Water is absobed
- Remaining waste material is stored as feces before being defecated
- No villi, but epithelial lining with crypts
Why are gut epithelial cells predisposed to become cancerous?
Intestine Epithelial Cells
- Cells are lost from the tips of SI villi and the lining of the S and L intestine.
- Groups of continually dividing cells at base of crypts.
- Stem cells produce undifferentiated cells.
- The transit amplifying compartment allows these non-differentiated cells to divide.
- Cells migrate upwards out of the crypt towards the epithelial lining surface.
What defence mechanism against cancer does the colorectal system possess?
Shedding/apoptosis of gut lining
- Almost all mutated cells will die soon after formation.
- This may be an escape mechanism from the development of a tumour.
- For a tumour to form, cells must escape apoptosis and avoid being shed.
How does a mutation within a colorectal cell lead to tumour formation?
- Mutation occurs in stem cell triggering APC loss.
- Cells no longer migrate out, instead remaining embedded in the crypt.
- A mass of continually dividing cells arises that are not shed and do not experience apoptosis.
- These cells divide and produce new stem cells in population.
- The adenoma grows and gives rise to more stem cells allowing for continuous growth.
What are the two types of inherited colorectal cancer?
- FAP (Familial Adenomatous Polyposis Coli)
- HNPCC (Hereditary Non-Polyposis Colorectal Cancer)
What are the key stages of colon tumour progression?
- The normal colorectal epithelium experiences a loss of APC and LOH (loss of heterozygosity) on Chr.5q
- This leads to a state of hyperplastic epithelium triggering DNA hypomethylation
- Masses of tissue develop and K-ras is activated. These adenomas (benign tumours) progress from Early → Intermediate → Late stage
- There is LOH Chr.18q and Loss of 18q TSG
- There is LOH Chr.17p and Loss of p53
- A carcinoma develops leading to invasion and metastasis

What is APC?
APC stands for Adenomatous Polyposis Coli
- APC is the ‘gatekeeper’ of cellular proliferation.
- APC is a large protein (> 300KDa)
- The protein can form oligos with itself, forming large complex structures.
- The middle third of the APC protein mediates β-catenin binding.
- The carboxy terminus contains a basic region that can bind microtubules and thus interact with cell scaffolding.
- Colon cancer cells with mutant APC have high levels of intracellular β-catenin.
- Mutations in APC, a tumour suppressor gene, can lead to the formation of benign polyps on the epithelial surface of the intestinal tract.

Where do the majority of APC mutations occur?
Most APC mutations occur within the mutator cluster region (MCR)
- The majority of these mutations lead to truncated proteins.
- Truncated proteins contain ASEF and β-catenin binding sites in the armadillo repeat domain.
- They lose β-catenin regulator activity (located in 20-aa repeat domain).
How many new cells do the crypts of the gut produce each day?
200-300 new cells/day
What is the purpose of the stem cells in the gut?
The stem cells produce replacement cells near the base of the crypt.
Why is the lining of the gut single cell layered?
The single layer allows for maximisation of absorption.
In a mouse how many cells does a single crypt contain?
~250 cells, one of which is a stem cell.
150 of these are proliferating.
How long does it take to replace the lining of the gut?
~3-4 days
What mechanisms must a mutated cell avoid in order to develop a tumour?
- Escape apoptosis
- Escape out-migration and shedding
If these cannot be avoided, the mutated cell is likely to be shed, digested and destroyed.
How many deaths does colorectal cancer cause in the UK a year?
15-20,000
What are colorectal polyps?
They are essential clusters of cells on the gut wall. These progress to form an adenoma, composed of abnormal glandular tissue.
What percentage of colorectal cancers are associated with dominant family history?
10%
What are hyperplastic polyps?
Hyperplastic polyps are those that will remain benign forever.
What is the difference between hyperplastic polyps and adenomas?
Hyperplastic polyps will always remain benign.
Adenomas have the potential to become malignant.
When a mutation occurs in the gut stem cells, what then occurs?
The mutation triggers APC loss. The cells, instead of migrating out, stay embedded in the crypt. This leads to a mass of continually dividing cells which are not shed and do not undergo apoptosis.
The cells divide and produce new stem cells in the population. As the adenoma grows, new stem cells are formed that are able to continually increase the number of cells.
What are the driving force behind colorectal cancer?
Polyps are the driving force behind CRC.
What are the 6 main processes that must be achieved in order for tumourigenesis to occur?
- Evading apoptosis
- Self-sufficiency in growth signals
- Insensitivity to anti-growth signals
- Tissue invasion and metastasis
- Limitless replicative potential
- Sustained angiogenesis
What does a loss of heterozygosity (LOH) at Chr.5q correspond with?
Loss of APC

What event triggers the transition in the gut, from Normal epithelium to Hyperplastic epithelium?
A LOH at Chr.5q, corresponds with the loss of APC and leads to the development of hyperplastic epithelium.

What LOH event triggers the transition of normal colon epithelium to hyperplastic epithelium?
LOH at Chr.5q, corresponding with the loss of APC.
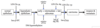
What triggers hyperplastic epithelium to develop into an early adenoma?
The hypomethylation of DNA.
What does the LOH at Chr.17p correspond with?

The loss of p53.
What are the two types of inherited colorectal cancer?
FAP (Familial Adenomatous Polyposis Coli)
HNPCC (Hereditary Non-Polyposis Colorcetal Cancer)
What is FAP?
FAP = Familial Adenomatous Polyposis Coli
- Arises through mutations in the APC gene
What is HNPCC caused by?
HNPCC (Hereditary Non-Polyposis Colorectal Cancer) is caused by genes involved in mismatch repair.
What type of inhertiance pattern does FAP demonstrate?
FAP is as Autosomal dominant inherited disorder.
What gene is mutant in FAP.
APC is the mutant gene in FAP
What follows the allelic mutation of APC?
LOH (loss of heterozygosity) often follows mutation in APC.
How can APC syndromes be recognised?
APC syndromes (FAP) have numerous florid (red) colonic polyps (100-2500).
Adenomas are frequent precursors of colorectal tumours.
What protein is described as the ‘gatekeeper’ of cellular proliferation?
APC - It keeps cell proliferation under control.
If APC is mutated, what happens to cellular levels of β-catenin?
Intracelluar levels of β-catenin rise, this can trigger cell proliferation.
Where do the majority of mutations occur within APC?
The majority of mutations in APC occur in the mutator cluster region (MCR)
The majority of these mutations lead to truncated proteins

What is the MCR in APC?
The MCR is the mutator cluster region
Mutations in APC often occur in the MCR, this can often lead to truncated proteins.
How can APC become truncated?
Mutations within the mutator cluster region (MCR) of APC can lead to truncated proteins. These truncated proteins contain ASEF and β-catenin binding sites in the armadillo-repeat domain.

What is Wnt signalling known as in Drosophila?
Wingless
What genes are included in Wnt Signalling?
Wnt, Porcupine, Dishevelled, Zeste white 3, Armadillo
How does Wnt interact with DSH? (Drosophila)
Wnt activates Dishevelled (Dsh) through binding with Frizzled (Fz). Fz transduces signal into the cell where it activates Dsh. Dsh negatively regulates ZW3.
Normally ZW3 makes the armadillo molecule unstable. It then gets degraded.
If ZW3 is functioning, armadillio is stabilised, it can then enter the nucleus and activate gene expression.

What is armadillo’s homologue in humans?
In humans the Drosophila armadillo protein is instead called β-catenin.
In the absence of Wnt signalling how does the Wnt pathway operate?
GSK-3 phosphorylates β-catenin, APC and Axin
This complex targets β-catenin for ubiquitination (targeted destruction) and it is broken down by the proteosome.
All the genes that β-catenin activates are not activated. β-catenin is degraded so it does not build up in the cytoplasm. LEF/TCF transcription factors with co-repressors, Groucho and CtBP repress gene transcription.

When Wnt signalling is activated how does the pathway operate?
Wnt binds to the Frizzled receptor
This triggers the activation of Dishevelled (likely by phosphorylation)
Dishevelled breaks apart APC-Axin-β-catenin-GSK3 complex
Repression of GSK-3 by Dsh involved GBP action and disassociation of GSK-3 from the complex.
β-catenin is not targeted for degradation, it accumulates in the cell, enters the nucleus, binds TCF and activates various different genes for transcription. The arrival of a ligand triggers gene expression.

In the presence of Wnt what breaks apart the APC-Axin-β-catenin-GSK-3 complex?
Dishevelled (Dsh), triggered by Wnt binding to Frizzled, breaks apart the complex.

What does Wnt bind to?
Wnt binds to Frizzled receptor, triggering the activation of Dishevelled (Dsh).
Why is APC essential for Wnt signalling?
APC holds the APC-Axin-β-catenin-GSK3 complex together.
If APC is not functioning the complex never forms and can never trigger the ubiquitination of β-catenin in the absence of Wnt. Thus cell switches on genes without the Wnt signal which would normally only activate in the presence of a Wnt signal.
How is c-Myc activated?
C-Myc is activated by β-catenin. C-Myc drives cellular prolfieration via the upregulation of Cdk4.
Cdk4 is required for the G1 cell cycle checkpoint. Increased levels of Cdk4 make lead to the phosphorylation of pRB.
What is c-Myc?
c-Myc is activated by β-catenin and drives cellular proliferation through the upregulation of Cdk4. Cdk4 is required for the G1 cell cycle checkpoint.
What activates c-Myc?
β-catenin
How does c-Myc influence cellular proliferation?
It upregulates Cdk4 which is required for the G1 cell cycle checkpoint. Upregulation of Cdk4 may lead to phosphorylation of pRB.
What is TCF-LEF?
TCF-LEF are DNA proteins that bind β-catenin.
High levels of β-catenin leads to complexes of β-catenin and TCF-4/LEF-1 and gene expression. This stimulates cell proliferation and/or inhibits apoptosis.
Where is TCF-4 expressed?
In the colonic epithelium. It is a β-catenin co-factor.
What must TCF-4 interact with in order to trigger transcription?
β-catenin

How do mutations in APC or β-catenin affect TCF-4?
These mutations activate TCF-4
If APC is functioning normally how is cell growth regulated?
β-catenin is degraded by the destruction complex through ubiquitination. β-catenin cannot activate transcription.

Are wnt signals active at the base of colon crypts?
Yes, β-catenin: Tcf/Lef target genes are induced by Wnts.
The stromal cells at the base of the crypts are the source of Wnt signalling, which drives cells to proliferate and migrate away. They continute to proliferate whilst receiving some Wnt signal. As they migrate away from the signal and toward the epithelial guy lining they stop dividing and start differentiating into normal, healthy, epithelial cells.

How does Wnt function differ in the crypts when APC mutations or other mutations interfere with the Wnt signals?
Stromal cells at the base of the crypt are the source of Wny signals. They act to drive cells to proliferate away and migrate to the epithelial gut lining.
Usually, cell cycle is arrested as cells migrate up from the crypts, β-catenin and Tcf/Lef are switched off.
In a damaged Wnt pathway these β-catenin and Tcf-Lef signals remain on. This leads to a build up of dividing cells that cannot leave the crypt - Polyp formation.

What is the role of ASEF?
APC can bind to ASEF (APC-stimulating guanine nucleotide exchange factor)
ASEF is activated in colorectal cancer cells containing APC.
Active ASEF decreases E-cadherin cell-cell adhesion and promotes migration.
How do ASEF and APC work together?
ASEF and APC cooperate to regulate cell movement. Active ASEF decreases E-cadherin cell-cell adhesion and promotes migration.
What is E-cadherin?
E-cadherin is responsible for cell-cell adhesion in epithelial cells. It requires a cytoplasmic catenin binding domain (either α,β or γ).
APC and ASEF act to maintain the actin cytoskeletal network.

In APC mutants how is E-cadherin affected?
In an APC mutant, ASEF interacts with E-cadherin resulting in reduced cell-cell adhesion. Thus there is no tight linkage between cells, migration is altered and occurs incorrectly.

How does mutated APC affect mitosis?
Cells lacking functional APC undergo aberrant mitosis. Normally, APC facilitates the interaction between microtubules and the kinetochore. In mutants this cannot occur so chromosomes with spindle abnormalities occur

In healthy cells, how does APC affect mitosis?
APC facilitates the interaction between microtubules and kinetochore.
APC binds and stabilises microtubules, it localises to clusters at the ends of the microtubules and is embedded within the kinetochore. It forms complexes with Bub1 and 3 and is an in vitro substrate for Bub kinases.

What is HNPCC otherwise known as?
Lynch syndrome
What is the difference in number of polyps between FAP and HNPCC?
APC (FAP) - >100 polyps
HNPCC - 10-50 polyps
What is the Amsterdam Criteria?
A diagnostic method for HNPCC
- 3 close relatives affected
- Two successive generations
- Age of diagnosis should be <50 for at least one
What other regions can HNPCC affect in addition to colon?
In addition to colon cancer, cancer of: Endometrium, Small bowel, Ureter, Renal pelvis
What are the distinct genetic regions implicated in HNPCC?
Chromosome 2p16/3p21
What are the two main genes implicated in the onset of HNPCC?
MSH2
MLH1
How are individuals with HNPCC diagnosed from molecular techniques?
Identify germ line mutations in MMR genes
At least 6 different proteins are required for a complete MMR system.
- MSH2, MLH1, PMS1, PMS2, MSH3, MSH6
MSH2 forms a heterodimer with either MSH6 or MSH3 and binds to mismatch.
The heterodimer is then bound by heterodimer of MLH1 and PMS2
The mutation rate in cells with MMR deficiency are 100-1000 fold greater than in normal cells.
MMR deficiency leads to common mutations in target genes including TGFβRII, MSH3, MSH6 and insulin like growth factor II receptor amongst others.
In which people do MSH6 and MLH3 gene mutations typically occur?
The mismatch repair genes, MSH6 and MLH3 feature less mutations than MSH2 and MLH1.
They are being increasingly identified and generalyl occur in clinically less typical HNPCC families with one or more of the following features.
- Late onset
- Frequent occurence of endometrial cancer
- Low degree of microsatellite instability
Mutations in MSH6 are common in microsatellite instability in many different tumours of diverse origin.
Why do mutations in TGFβ occur?
TGFβ contains a run of As
This run of identical bases is difficult to replicate correctly if there are problems with mismatch repair.
Deletions are very common - leads to a mutant form which has a premature STOP. This results in the formation of a truncate receptor, the TGFβ signal tends to shut cells down and stop division.
If the TGFβ receptor is mutated there will not be a stop growing signal. It will continue dividing.
90% of colorectal cancers with MSI (microsatellite instability) have truncated TGFβ receptor.

What is the function of TGFβ?
TGFβ signalling inhibits the growth of normal epithelial cells.
If a mutation occurs the TGFβ receptor is truncated and thus cannot respond to antigrowth signals.

How does HNPCC arise in individuals without MMR mutations?
2/3 of all clinically typical HNPCC families show MSI (microsatellite instability) in tumour tissue and display germline mutations in MMR genes.
In the other 1/3, mutations are reported in TGFβ receptor, E-cadherin, specific APC variants.
In addition, mutations have been identified in two novel genes in two separate families - HMPS and CRAC1.
What is the most common treatment for colorectal cancer?
Surgery
What are the common treatment methods for colorectal cancer?
Surgery
Chemotherapy
Radiotherapy
How can curcumin (turmeric) affect cells with mutated APC/B-catenin?
Curcumin activates caspase-3 in the cells which disrupts wnt signalling and targets the cell for degradation.

How can curcumin affect cell-cell adhesion/apoptosis?
Curcimin degrades B-catenin and APC and E-cadherin. This leads to cells breaking up. The inappropriately migrated cells that are forming polyps are shed as a result causing the cells to be replaced.


