Cholinomimetics Flashcards
(49 cards)
Describe the synthesis, release and metabolism of acetylcholine.
Synthesis
Acetyl coA + choline → Ach + coA
- Catalysed by choline acetyltransferase (CAT)
Release
- ACh packaged into vesicles
- AP triggers Ca2+ influx into pre-synaptic terminal
- This stimulates vesicle exocytosis and release of ACh into synpatic cleft
Metabolism
-
Acetylcholinesterase, present in the synaptic cleft, breaks down ACh:
- ACh → choline + acetate
- Choline and acetate can be recycled back into the pre-synaptic nerve terminal so they can be used to make more ACh
What is the difference between muscarinic and nicotinic effects?
Muscarinic effects are those that can be replicated by muscarine (muscarinic receptor agonist)
Nicotinic effects are those that can be replicated by nicotine (nicotinic receptor agonist)
NOTE: ACh acts on both muscarinic and nicotinic receptors
What can be given to abolish muscarinic effects?
Atropine (competitive muscarinic antagonist)
After atropine blockade of muscarinic actions, what can large doses of ACh induce?
Large doses of ACh can induce similar effects to those caused by nicotine
- Because the muscarinic receptors are blocked so therefore you get more ACh binding to the nicotinic receptors
- These nicotinic receptors also respond to nicotine therefore nicotine and ACh would have similar effects in this case
Which branch of the ANS do muscarinic actions correspond to?
Parasympathetic nervous system
- Muscarinic receptors are present on parasympatheitc effector organs
- EXCEPTION: sweat glands have muscarinic ACh receptors but have sympathetic stimulation (i.e. post-ganglionic neurone terminated in sympathetic trunk)
State where you would find the different muscarinic receptor subtypes.
M1
- Salivary glands
- Stomach
- CNS
M2
- Heart
M3
- Salivary glands
- Bronchial/visceral smooth muscle
- Sweat glands
- Eye
M4 - CNS
M5 - CNS
NOTES:
- M1, M2 and M3 are the main receptor subtypes
- Muscarinic receptors are generally excitatory (stimulates muscle contraction, secretion) except for on the heart (decreases heart rate and contractlilty)
What type of receptor are all muscarinic receptors?
G-protein coupled receptors
What is the difference in the G-protein receptors of M1, M3 and M5 compared to M2 and M4?
M1, M3 and M5 = Gq protein linked receptors (odds)
- They stimulate phospholipase C (PLC) which converts PIP2 increases IP3 and DAG
- Second messengers: IP3 and DAG - increased
M2 and M4 = Gi protein linked receptors (evens)
- They inhibit adenyl cyclase which would convert ATP to cAMP
- Second messenger: cAMP - decreased
Describe the structure of nicotinic receptors. What determines its ligand binding properties?
Nicotinic receptors are ligand-gated ion channels (i.e. ion channel opens when ligand binds)
Nicotinic receptors consist of 5 subunits:
- α = alpha
- β = beta
- γ = gamma
- δ = delta
- ε = epsilon
The combination of subunits determines its ligand binding properties.
What are the two main types of nicotinic receptor? Describe their subunit composition.
Two main nACh receptor types:
- Muscle
- Autonomic ganglion
Muscle - 2α, β, δ, ε
Ganglion - 2α 3β (similar structure to this for nACh receptors found in the CNS)
What is the relevance of having two nicotinic receptor subtypes in terms of pharmacology?
- This difference in subunit combination gives slightly different ligand-binding properties
- This means that drugs can be developed which are more selective for one receptor subtype than the other (e.g. drugs which act on the NMJ)
REMEMBER: The drugs are never 100% selective so they also have the potential to affect the other (unwanted) receptor subtype
How do the effects of acetylcholine on nicotinic receptors compare to its effects on muscarinic receptors?
The effects of acetylcholine are relatively weak on nicotinic compared to muscarinic
- Relatively weak - i.e. you need more ACh to stimulate nicotinic receptors
- This is probably because ACh has a lower affinity for nicotinic receptors so you need more collisions between receptor ligand in order for proper binding to take place
- Reduced chance of proper binding per collision
What three effects does muscarinic stimulation have on the eye?
Contraction of the ciliary muscle
- Accommodates for near vision
- It does this by making the lens thicker so there is more refraction of light from nearby objects onto the retina
Contraction of sphincter pupillae (circular muscle of the iris)
- This constricts the pupil (miosis) and increases drainage of intraocular fluid
Lacrimation (tears)

What is glaucoma?
Sustained raised intraocular pressure – this can cause damage to the optic nerves and retina which can lead to blindness
How can a muscarinic agonist be used to treat glaucoma?
- In angle-closure glaucoma, the angle between the iris and cornea is too small (closed)
- This which blocks the flow of the aqueous humour out of the iris
- Contraction of sphincter pupillae (cicular muscle of iris) opens up this angle
- This provides a pathway for aqueous humour drainage via the canals of Schlemm → reduced intraocular pressure
REMEMBER: This is only relevant for angle-closure glaucoma (there are other types of glaucoma which have a different cause for the raised intraocular pressure)
NOTE: Aqueous humour drains through the trabecular meshwork into the canals of Schlemm
Describe, in detail (including the mechanism), the muscarinic effects on the heart.
- Decrease in intracellular Ca2+ in heart muscle decreases myocardial contractility
- ↓ contractility → ↓ force of contraction → ↓ CO
- This is because Ca2+ is required to bind to the muscle fibre to stimulate it to contract
- Less Ca2+ binding means less actin-myosin cross bridges and hence reduced force of contraction
- Increasing K+ efflux from the conductive tissue in the heart decreases heart rate
- This is because it hyperpolarises (more -ve) the cells meaning that it takes longer to reach the threshold potential for generating an AP
- Reduced AP frequency = reduced HR
NOTE: receptors present in atria (i.e. atrial muscle) and nodes (i.e. SAN and AVN)
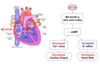
Describe the muscarinic effects on the vasculature.
- Most blood vessels do NOT have parasympathetic innervation
- Acetylcholine acts on vascular endothelial cells to stimulate NO release via M3 ACh receptor
- NO induces vascular smooth muscle relaxation → vasodilation
- Result is a decrease in TPR
NOTE: This mechanism is more relevant to the clinical use of cholinomimetics than normal physiology
Summarise the muscarinic effects on the cardiovascular system.
- Decreased heart rate (bradycardia)
- Decreased cardiac output
- Due to decreased atrial contraction
- Reduced atrial contraction → reduced blood volume emptied from atria into ventricles → reduced blood volume ejected from ventricles - SV
- Vasodilaation and reduced TPR
- Due to stimulation of NO production
All of these combined can lead to a sharp drop in blood pressure
Describe the muscarinic effects on non-vascular smooth muscle.
It is the opposite of muscarinic effects on vascular smooth muscle
It causes CONTRACTION of non-vascular smooth muscle
- Lungs – bronchoconstriction → diffuculty breathing
- GI tract – increased peristalsis (motility) → GI pain
- Bladder – increased bladder emptying
Describe the muscarinic effects on exocrine glands.
Increases secretions from exocrine glands
- Increased salivation
- Increased lacrimation (tears)
- Increased bronchial secretions (e.g. mucus) → diffuculty breathing
- Increased GI secretions (including gastric HCl production) Increased sweating (SNS-mediated)
What are the two types of cholinomimetic drug?
Directly Acting – muscarinic receptor agonists
Indirectly Acting – acetylcholinesterase inhibitors (anticholinesterases)
State two types of muscarinic receptor agonists and give an example of each.
Choline Esters – Bethanechol
Alkaloids - Pilocarpine
Describe the selectivity of pilocarpine.
Non-selective muscarinic receptor agonist
- It stimulates ALL muscarinic receptor subtypes
What is the half-life of pilocarpine?
Approximately 3-4 hours
- Relatively long half life
- This is due to its good lipid solubility
- This makes it harder to be cleared from the plasma (undergo renal excretion)
- Its lipid solubililty means that it is reabsorbed back into the blood from the urine via the kidney tubular cells (lipid membranes)