ch18 Flashcards
(70 cards)
Rutherford experiment disproved which model for the atom?
describe the model
The Thompson model or plum pudding model
atoms were a plum pudding with electrons embedded throughout a positive sphere
how do you investigate the structure of an atom
with scattering experiments
particles are accelerated to a high energy and directed at a target
Describe Rutherford’s experiment and his results
A stream of alpha particles from a radioactive source was fired at a very thin gold foil.
The angles at which the particles were scattered were recorded
some passed straight through (so most atom is empty space)
but a few of the alpha particles bounced right back from the foil being scattered at angles greater than 90 degrees
What could Rutherford conclude after his experiment?
The atom is mainly empty space.
the core must be massive on an atomic scale to deflect alpha particles through large angles, but its much smaller than an atom as very few particles deflected through more than 90 degrees
alpha particles deflected due to electric repulsion between positively charged alpha particles and positive core in gold atoms.
The centre of the atom must have a large, positive charge. Rutherford named this the nucleus.
The nucleus must be tiny, but massive (mass).

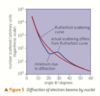
1 in how many alpha particles were turned back and what does this suggest about the size of a nucleus
diameter of atom
diameter of nucleus
1 in 10000 turned around so nucleus is 10 times smaller than the atom
diameter of atom is 10-10m
diameter of nucleus therefore is 10-14 m

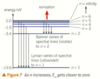
what happens to the deflection angle if the alpha particles are slowed done
how is scattering affected if the nuclei has less electric charge
relationship between scatter angle and inverse square law
if alpha particles are slowed down more would be deflected at greater angles since the nucleus would be able to turn them back more easily
nuclei of smaller electric charge scatter alpha particles less strongly
the pattern of number of alpha particles scattered at different angles fits the pattern expected from the inverse square law for electric repulsion

alpha particles are highly ionising and so will lose their energy over a short distance ionising air particles
by evacuating the apparatus (removing air particles) you cen minimise collisions and therefore get an accurate measure of the deflection angles
kinetic energy of alpha particle at its closest approach
0 kinetic energy
what is electrical potential energy equal to at a large distance from the nucleus
at a large distance from the nucleus, alpha particle electrical potential energy is equal to its initial kinetic energy
equation for initial kinetic energy
charge on alpha particle
charge on gold particle
initial kinetic energy = electrical potential energy = kQq / r
alpha: 2e = 2 *(1.6*10-19)
gold: 79e = 79 * (1.6*10-19)

force that a particle experiences near a nucleus
F = KQq / r²

What did Rutherford discover about charge at the centre of the atom?
Some of the alpha particles were deflected through large angles, so the centre of the atom must have a large positive charge to repel them.
What value do you need when you are estimating the distance of closest approach of an alpha particle that has been fired at a gold nucleus?
The alpha particles initial kinetic energy


What value do you need to find the charge of the nucleus?
The atom‘s proton number, Z
how are electrons similar to alpha particles
electrons are scattered by the nucleus in the same way alpha particles are, but the force is attractive this time.
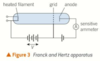
how to produce high energy electrons
using particle accelerators
one application of particle accelerators
dental x-ray tubes are particle accelerators
electrons boiled off heated wire and accelerated towards positive target
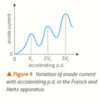
describe the change in energy for electrons in dental x-ray tubes
rearrange to give velocity
electrons lose electrical potential energy (qV) and gain kinetic energy (1/2mv²)
qV = 1/2mv²
can rearrange for v (velocity)
V = accel voltage
q = charge
m = mass of electron
limitations of momentum equation
accurate at low speeds but not at high speeds
when v << c
einstein momentum equation
momentum = relativistic factor * mass * velocity
total energy of free particle
kinetic energy + rest energy
= relativistic factor * m * c²
equation for energy when particle at rest
relativistic factor is 1 at rest so
Erest = mc²
what is rest energy equivalent to
mass
Erest = m