Ch 10, 11, 13, 14, 16 Flashcards
Nucleic Acids, Fats, Glycolysis, CTA
Calculate the standard free-energy change of the reaction catalyzed by the enzyme phosphoglucomutase given that starting with 20 mM glucose 1-phosphate and no glucose 6-phosphate, the final equilibrium mixture at 25ºC and pH 7.0 contains 1.0 mM glucose 1-phosphate and 19 mM glucose 6-phosphate.
Does the reaction in the direction of glucose 6-phosphate formation proceed with a loss or a gain of free energy?

First, calculate the equilibrium constant.
Then, calculate the standard free-energy change

What is the difference between ∆Gº’ and ∆G?
How are they related?
The standard transformed free energy change (∆Gº’) tells us in which direction and how far a given reaction must go to reach equilibrium when the concentration of each component is 1.0 M, the pH is 7.0, the temperature is 25ºC, and the pressure is 101.3 kPa (1 atm). Thus ∆Gº’ is a constant.
The actual free energy change/Gibbs free energy (∆G) is a function of reactant and product concentrations and of the temperature prevailing during the reaction. The ∆G of any reaction proceeding spontaneously toward its equilibrium is always negative, becomes less negative as the reaction proceeds, and is zero at the point of equilibrium.
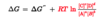
actual free-energy change (∆G) = 0 vs standard free-energy change (∆Gº’) = 0
∆G=0: implies that the reaction is at equilibrium (no more work is done by the reaction in either direction)
∆Gº’ = 0: implies that the equilibrium constant (the concentrations of reactants and products at equilibrium) is 1
What are the conditions in which metabolic reactions with a large positive ∆Gº’ can still be exergonic?
A reaction with a positive standard-free energy change can go in the forward direction if ∆G is negative.
Immediate removal of products to keep the ratio of products to reactants well below 1 so that the term RTlnQ has a large, negative value

FRAP
Fluorescence Recovery After Photobleaching
allows us to monitor lateral lipid diffusion by monitoring the rate of fluorescence return; the diffusion coefficient of lipid in the leaflet can be determined
rates of lateral diffusion are high (up to 1µm/sec); a lipid can circumnavigate E. coli cell in one second
Membrane Rafts
there’s also different lipid distribution within a membrane (not only between membranes)
lipid rafts
- contain clusters of glycosphingolipids with longer-than-usual tails (more opportunity for van der Waals interaction, giving more stability)
- are more ordered
- contain specific doubly or triply acylated proteins
- allow segregation of proteins in the membrane
Caveolin
when several caveolin dimers are concentrated in a small region (a raft) they force a curvature in the lipid bilayer, forming a caveola
flattening of caveolae allows the plasma membrane to expand (increase surface area)

other modes (besides caveolin) of membrane curvature
- a protein with intrinsic curvature and a high density of positive charge on its concave surface
- a protein with one or several amphipathic helices inserted into the outer leaflet of the bilayer
- proteins with BAR domains can polymerize into a superstructure that favors and maintains the curvature
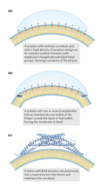
membrane fusion
membranes can fuse with each other without losing continuity
fusion can be spontaneous or protein-mediated
examples of protein-mediated fusion: entry of influenza virus into the host cell and release of neurotransmitters at nerve synapses
Why is passive diffusion of polar molecules across membranes unfavorable?
involves desolvation and thus has a high activation barrier
What are the 3 classes of transport systems?
- uniport: facilitated diffusion
- symport (cotransport): secondary active transport
- antiport (cotransport): secondary active transport
Glucose transport
- glucose in blood plasma binds to T1
- conformational change from T1 to T2
- glucose is released from T2 into the cytoplasm
- the transporter returns to T1

There are multiple glucose transporters
A Na+-glucose symporter (intestinal lumen) and a glucose uniporter (blood) operate on opposite sides of epithelial cells
cells can also have asymmetry with distinct proteins confined to one side
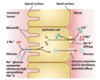
Bicarbonate transporter
antiporter: chloride-bicarbonate exchanger
maintains the electrochemical potential across the membrane

two types of active transport
a) primary active transport: energy released by ATP hydrolysis drives solute movement against an electrochemical gradient
b) secondary active transport: a gradient of an ion has been established by primary active transport; movement of S1 down its gradient now provides energy to drive cotransport of a second solute against its electrochemical gradient
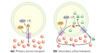
ABC Transporters
a large family of ATP-driven transporters that pump ions and molecules against a concentration gradient
located in the plasma membrane, in the ER, mitochondria, and lysosomes
CFTR protein of the plasma membrane (airway epithelium):
- ion channel for Cl
- when it doesn’t work, cystic fibrosis; mucus accumulates, entrapping bacteria that results in infection of lungs and airway
Proton pumps
ATPase uses ATP to pump protons through the membrane
the energy of the proton gradient can be used to make ATP (in chloroplast and mitochondrial membranes) by ATP synthase
aquaporins
allow rapid water passage through membranes
size restriction: the pore narrows at His 180 to a certain diameter, limiting the passage of molecules larger than H2O.
electrostatic repulsion: the positive charge of Arg 195 repels cations, including H3O+
water dipole reorientation: the helices with Asn-Pro-Ala are oriented with their positively charged dipoles pointed at the pore to force H2O to reorient as it passes through; this breaks up hydrogen-bonded chains of water molecules and prevents proton passage by proton hopping

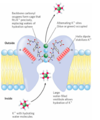
K+ channels and their specificity
at the inner and outer plasma membrane surfaces, the entryways to the channel have several negatively charged amino acid residues
the pore is a specific diameter that allows a specific ion and its water shell to pass through the channel
How do major nucleotide mutations occur?
naturally
radiation
reactive chemicals
Accumulation of nucleotide mutations is linked to what?
aging and carcinogenesis
What are the two types of naturally occurring mutation reactions?
deamination and depurination
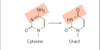
deamination
very slow reaction; occurs one in 107 cytosine residues per day (~100 spontaneous events per day); adenine to guanine occurs much slower (1/100th the rate of C to U)
cytosine is mutated to uracil; uracil is recognized as foreign in DNA and removed by a repair system
thymine allows for long-term storage of genetic information; otherwise, AU not recognized as mutation, and eventually GC will be eliminated
depurination
N-glycosidic bond is hydrolyzed; the base is lost, creating an abasic site
purines are lost at a higher rate than pyrimidines (10,000 purines lost per day in one mammalian cell); depurination of RNA is much slower and less physiologically significant
proteins recognize damage and fill in gap