Cells and half cells Flashcards
Explain what a copper half cell is and the equilibrium that occurs in it.
A copper half cell comprises a solution containing Cu2+(aq) ions ( oxidation state +2) into which is placed a strip or rod of copper metal (oxidation state 0).
An equilibrium exists at the surface of the copper between these oxidation states of copper:
Cu2+(aq) + 2e- ⇔ Cu(s)
Describe, explain and draw a cell.
A simple electrochemical cell can be made by connecting together two half cells with different electrode potentials:
- One half cell releases electrons and the other half cell gains electrons.
- The wire connects the two metal allowing electrons to be transferred between the two half cells. The voltmeter has a high resistance and is used to minimise the current that flows. If a small bulb were to replace the voltmeter, the bulb would light up.
- The salt bridge connects the two solutions, allowing ions to be transferred between the half cells. A simple salt bridge can be made out of a strip of filter paper soaked in an aqueous solution of an ionic compound that does not react with either of the half-cell solutions. Usually aqueous KNO3 or NH4NO3(aq) is used.

In a cell what does the reading of the voltmeter tell you?
The reading on the voltmeter measures the potential difference of the cell - this measures the difference between the electrode potentials of the half cells.
Draw a hydrogen half cell.
- Explain all the standard conditions for the half cell setup.
A hydrogen half cell comprises hydrogen gas, H2, in contact with H*(aq) ions.
- Hydrochloric acid, HCl(aq), of concentration 1 mol dm-3, as the source of H*.
- hydrogen gas, H2(g), at 100KPa ( 1 atmosphere) pressure.
- An inert paltinum electrode to allow electrons to pass into or out of the half cell via a conecting wire.

Explain the use of the platinum electrode in a hydrogen half cell.
Platinum electrode is place in the solution so that it is in contact with both H2(aq) and H*(aq) ions. The platinum is inert and does not react at all - its sole purpose is to allow the transfer of electrons into and out of the half cell via a connecting wire. The surface of the platinum electrode is coated with platinum black, a spongy coating in which electrons can be transferred between the non-metal and its ions.
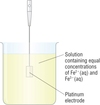
Draw and describe a metal ion/metal ion half cell such as Fe3+ and Fe2+.
- Fe2+(aq) + e- ⇔ Fe3+
A standard Fe2+(aq)/Fe3+(aq) half cell is made up of:
- A solution containing Fe2+ and Fe3+ ions with the same concentrations (‘equimolar’).
- An inert platinum electrode to allow electrons to pass into or out of the half cell via a connecting wire.