BIOCHEM: Nucleotides, carbs & lipids Flashcards
How can we prove that a pathway works?
- Look at the pathway, an important/unique step from that pathway must be inhibited to see if the effects are changed.
nucleoside
is a pentose (five-carbon) sugar linked to a nitrogenous base on the 1′ carbon by a covalent glycosidic bond.

nucleotide
consists of a nucleoside attached to one or more phosphate groups by a phosphoester bond.

If a pentose has a hydroxyl group at the 2′ carbon, it is __________
ribose
If the pentose has a hydrogen at the 2′ carbon it is a ________
deoxyribose
What are hydrogen bond acceptors?
Hydrogen bond acceptors are electronegative atoms (nitrogen or oxygen) that have at least one lone pair of electrons
what are hydrogen bond donors
are hydrogen atoms bound to electronegative atoms.
For each nucleotide of thw watson & crick model, how many donors & acceptors does each pair have?

Melting Temperature Tm of DNA & whats the proportion to that of IMF?
- the temperature at which 50% of the double helices in solution have been seperted into single strands
- it is determined by the strength of intermolecular forces holding the strands together (an inc IMF(hydrogen bonding)=inc Tm bc more energy is required to disrupt them)
- DNA molecules with high GC content have higher melting temperatures than those with low GC content.

Enantiomers
- compounds with the same chemical formula but DIFFER AT EVERY STEREOCENTER
- Example: L-glucose & D-glucose on fishcer projections

Carbs:
what determines the absolute configuration (D or L)?
the chiral carbon furthest from the carbonyl group determines the absolute configuration L or D of the sugar
Carbs:
Stereoisomers (aka optical isomers):
two or more compounds with identical molecular formulas and arrangements of atoms that only differ in the spatial arrangement of their atoms; they have non-superimposable mirror images.
Carbs:
Enantiomers
- the same sugars in different optical families (such as D-glucose and L-glucose).
- differ at every chiral carbons
- no internal planes of symmetry

The number of possible stereoisomers of a compound can be calculated by:
2n
n=the number of chiral carbons present
Carbs:
Fischer projections, how to determine L or D?

Carbs:
Diastereomers
- are two sugars that are in the same family (that are stereoisomers), but are not identical and are not mirror images of each other.
Carbs:
Epimers
- are a special subtype of diastereomers that differ in configuration at exactly one chiral center.
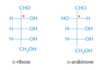
- D-ribose and D-arabinose are epimers (at carbon number 2)
Anomers can exist only in:
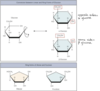
- in cyclized sugars (furanose or pyranose) and _differ at the anomeric carbon_
-
Anomeric carbon: A carbon derived from the carbonyl carbon (the ketone or aldehyde functional group) of the open-chain form of the carbohydrate molecule
- α form: OH on the bottom
- β form: OH on the top
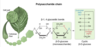
mutarotation
- The opening and closing of sugars repeats continuously in an ongoing interconversion between anomeric forms and is referred to as mutarotation
- Cyclic compounds undergo mutarotation, in which they shift from one anomeric form to another with the straight-chain structure


