Atoms, Molecules and Ions Definitions and Formulas Flashcards
Who is Democritus?
- Greek philosopher lived 460 - 370 B.C.
- Proposed that elements are composed of tiny, descrete, indivisible particles called atomos
- His ideas were rejected for 2000 years.
Who is Robert Boyle?
- Englishman lived 1627 - 1691
- Studied chemistry and carried out rigorous chemical experiments
- Defined elements as a substance that cannot be chemically broken down further
- Suggest that a substantial number of different elements may exists.
Who is Joseph Priestly?
- Lived 1733-1804
- Prepared and isolated oxygen gas in 1774 by heating mercury(II) oxide (HgO)
2HgO → 2Hg + O2
Who is Joseph Proust?
- lived 1754 - 1826
- Law of definite proportion:
- a pure substance, whatever its source, always contain definite or constant proportion of elements by mass.
Who is John Dalton?
- English Chemist, lived 1766 - 1844
- Used the inductive process by combining Lavoisier and Proust’s theories to arrive at the atomic theory in 1808
Dalton’s Atomic Theory
An explaination of the nature of matter, in terms of different combinations of very small particles.
- Elements are made up of tiny, indivisible particles called atoms
- Atoms of the same element are similar in mass and size
- Atoms of differenct elements have different masses and sizes
- Chemical compounds are formed by the combination of two or more atoms of different elements.
- Atoms combine to form compounds in simple numerical ratios (1:1, 2:2 etc…)
- Atoms to two elements may combine in different ratios to form more than one compound.
Modifications to Dalton’s atomic theory
- Atoms are composed of subatomic particles.
- Not all atoms of a specific element have the same mass (isotopes)
- Atoms under special circumstances can be decomposed.
Postulates to Dalton Atomic Theory
- All matter is composed of individual atoms. An atom is an extremely small particle of matter that retains its identiy during chemical reactions
- Element is a type of matter composed of only one kind of atom, each atom of a given kind having the same properties
- Compound is a type of matter composed of atoms of two or more elements chemically combined in fixed proportions.
- Chemical Reaction consists of the rearrangement of the atoms present in the reacting substances to give new chemical combinations present in the substances formed by the reaction.
Atomic Symbol
- One or two letter notation used to represent an atom corresponding to a particular element.
- Dalton used spheres to model atoms.

Law of multiple proportions
Deduced from Dalton’s Atomic theory, states that when two elements form more than one compound, the masses of one element in these compounds for a fixed mass of the other element are in ratios of small whole numbers.
e.g. nitrogen and oxygen can form in either a 7:8 ratio for NO
or a 7:16 ratio to form NO2
Structure of the Atom
- Two kinds of particles make up an atom
- Nucleus
- Central core, positively charged and contain most of the atom’s mass
- Electron
- Very light, negatively charged particle outside the nucleus.
- Nucleus
Discovery of the Electron
- English physicist, Joseph John Thomson (1856-1940), used cathod ray tubes to discover electrons and he also could caclulate the ratio of the electron’s mass to the electrons electric charge.
- American, RA Millikan (1868-1953), discovered the charge of the electon’s charge by oberving how a charged drop of oil falls in the presence and absence of an electric field. (1.602x10-19 coulombs)
Who is Ernest Rutherford?
- British physicist, lived 1871-1937
- Put forth the nuclear model of the atom in 1911
- Discovered that most of the mass is in the nucleus, about 99.95% or more (atom has diameter of 10-10m, the nuleus has the diameter of 10-15)
Proton
nuclear particle having a positive charge equal to that of the electron and a mass more than 1800 times that of the electron.
Atomic Number (Z)
The number of protons in the nucleus of the atom

Element
Substance whose atoms all have the same atomic number.
Who is James Chadwick?
British Physicist, lived in 1891-1974 who discovered the neutron.
Neutron
Nuclear particle having a mass almost identical to that of the proton but with no electric charge.
Mass Number
- Also known as Nucleons
- Total number of protons and neutrons in the necleus

Nuclide
- an atom characterized by a definit atomic number and mass number
- atoms are usually electrically neutral because of the equal amount of protons and electrons.
Isotopes
- Atoms whose nuclei have the same atomic number but different atomic mass numbers
- This is due to same number of protons but different number of electrons.
Atomic Mass Unit (amu)
mass unit equal to exactly one-twelfth the mass of carbon-12 atom.
Atomic mass
- also known as weighted average atomic mass
- average atomic mass for the naturally occurring element expressed in atomic mass units
- the weighted average of the isotopic masses of the element’s naturally occurring isotopes.
Fractional Abundance of an isotope
- The fraction of the total number of atoms that is composed of a particular isotope.
- Percent = fraction x 100%
- fraction = percent/100%
- contribution of isotope = fractional abundance x mass of isotope.
Periodic Table
- A tabular arrangement of element in rows and columns, highlighting the regular repetition of properties of the elements.
- Elements are now arranged by atomic number rather than atomic mass.
Period
The elements in any horizontal row of the periodic table.
(7 Rows)
Group
- the element in any one column of the periodic table
- elements in a given group have similar chemical properties
- Main group elements (Group A) consists of the first two column and the last 6 column of the periodic table
- The Trainsitional elements (group B) consist of the middle 10 rows of the periodic table.
Metal
- Substance or mixture that has a characteristic luster, or shine an is generally a good conductor of heat and electricity.
- Found on the left side of the periodic table
non-metals
element that does not exhibit the characteristics of a metal.
Found on the right side of the periodic table.
Metalloids (Semimetal)
- An element having both metallic and nonmetallic properties.
- semiconductor is a poor conductor of electricity at room temperature, but are good conductors at higher temperature.
Chemical Formulas
- is a notation that uses atomic symbols with numerical subscripts to convey the relative proportions of atoms of the different elements in the substance.
- example: H2SO4 rather than HHSOOOO

Molecule
- Definite group of atoms that are chemically bonded together - that is, tightly connected by attractive forces.
- Uncharged unit of a compound formed by the combination of two or more atoms bonded by covalent bonds
- Are the smallest characteristic entities of a molecular compound.
Molecular Formula
Gives the exact number of different atoms of an element in a molecule
e.g. C12H22O11
Structural Formula
Show how the atoms are bonded to each other in the molecule

Polymers
Very large molecules that are made up of a number of smaller molecules repeatedly linked together.
Monomers
Small molecules that are linked together to form the polymer.
Ion
- Positively or negatively charged atom or group of atoms.
- Results from the loss or gain of electons
Anion
Negatively charged ion from the gain of electrons.
e.g. Cl- or SO42-
Cation
positively charged ion from the loss of electrons
e.g. Na+ or Ca2+
Ionic compound
- Compound composed of cations and anions
- Ionic compounds are held together by attractive forces that exist between positively and negatively charged ions.
Formula Unit
- Group of atoms or ions explicitly symbolized in the formula.
- all substances, including ionic compounds, are electrically neutral

Organic Compounds
Molecules of carbon and other elements like hydogen, oxygen and nitrogen.
Hydrocarbons
Those compounds containing only hydrogen and carbon
Functional group
the reactive portion of a molecule that undergoes predictable reactions.
Hydrates
- contain water molecules weakly bound in crystal.
- an ionic compound in which the formula unit includes a fixed number of water molecules associated with cations and anions.
Chemical Equations
- Representation of chemical reactions
- shorthand description of a chemical reaction, using symbols and formulas to represent the elements and compounds
- Reactant - Starting substance
- Product - resulting substance
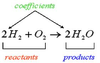
What are the symbols used in chemical equations?

What is the reason to balance a chemical equation?
- Based on the atomic theory and the law of conservation of matter and mass, atoms cannot be created nor destroyed.
- The number and kind of atom must be equal

What are the steps in balancing an equation?
- Write the skeleton equation of what took place.
- H2 + O2 ⇒ H2O
- Use coefficients to indicate how many formula units are needed to balance the equation.
- 2H2 + O2 ⇒ 2H2O
- Do not add subscripts
- Reduce coefficients to small whole numbers by dividing by a common divisor.
- Check answer. Every atom must be balanced on both sides.


