Anemia Flashcards
(47 cards)
How are erythrocytes produced?
- An uncommitted pluropotential stem cell is signaled to become a RBC by erythropoietin
- It leaves the bone marrow and enters the blood stream as a reticulocyte (baby red blood cell)
- at this stage it still has ribosomes and is making Hgb
- Becomes erythrocyte- it has achieved final size and shape
- no longer has any nucleus or ribosomes and can no longer make Hgb

Where are RBCs made throughout the lifespan?
- as an embryo, blood is made in the yolk sac
- then it is made in the liver until after birth
- After birth the bone marrow takes over, but if there is ever an issue, liver and spleen can become blood makers again and help pick up the slack
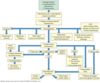
What are the progression and manifestations of anemia?
(chart)
- Some kind of event results in decreased RBCs and Hgb (decreased synthesis of blood, bleeding, increased destruction of RBCs, etc)
- decrease in RBCs leads to decreased carrying capacity of O2
- this causes tissue hypoxia
- Compensatory mechanisms kick in, eventually leading to hyperdynamic circulation

What are the compensatory mechanisms for the tissue hypoxia that is caused by anemia?
(chart)
- increased HR (increases the O2 demand for the heart)
- Capillary dilation
- Renal- Releases Epo
- RAAS
- Increased DPG in cells (causes righ shift of oxy-hemoglobin dissociation curve)
How does erythropoietin regulate erythropoiesis?
- O2 sensors are in the kidney (b/c it works at about the same rate and needs the same O2 almost all the time)
- if O2 is low after going through tubules, epo is released

What is macrolytic anemia?
What can cause it?
- MCV > 100 fl- (aka megaloblastic anemia)
- there is a problem with DNA synthesis so each RBC ends up larger than normal
- don’t have enough nucleotides so it takes longer to make nucleus
-
b12 deficiency- (pernicious anemia)- lack of IF
- gastric parietal cells secrete IF; may be genetic or acquired
-
Folate deficiency- common in alcoholics
- most common cause of macrolytic anemia
- drugs that inhibit DNA synthesis- cancer chemo, reverse transcriptase inhibitors
What is microcytic anemia?
What causes it?
- MCV < 80 fL (smaller than usual)
- hypochromic- less color
- Problem with Hgb synthesis, can’t make enough
-
Iron deficiency- adults: blood loss; children: nutritional deficiency
- the only way to lose Fe once you have taken it in is by bleeding
-
Thalassemia- genetic defect in alpha-globin or beta-globin
- not enough of one of the globins needed to make Hgb
What is normocytic anemia?
What are the normocytic anemias caused by decreased production (low RI)?
What causes it?
- normal color, normal size; decreased production
- low Reticulocyte index (RI)
- anemia of chronic renal disease- EPO deficiency
- anemia of chronic disease- Fe is taken up by macrophages, to “keep it away from bugs”
-
sideroblastic anemia- defect in iron handling causes dysfunctional Hgb
- Genetic
- acquired- lead poisoning
- Myelofibrosis- marrow replaced with fibrosis (pancytopenia)
-
Aplastic anemia- marrow replaced with fat (pancytopenia)
- genetic- congenital aplastic anemia
- acquired- bone marrow toxicity from drugs
What is normocytic anemia?
What are the normocytic anemias caused by increased erythrocyte turnover (high RI)?
What causes it?
- normochromic
- increased erythrocyte turnover
- High RI
- All are genetic- thought to be protective against malaria
- Caused by Hemolytic anemia
- membrane defect
- metabolic defect
- Hgb defect
- Also caused by hemorrhagic anemia
How do you correct the reticulocyte count for the degree of anemia
What is this used for?
- It is used to understand if bone marrow is making enough RBCs to try to make up for the anemia
- RI = reticulocyte count * (Hct/45%)/days as reticulocyte
- RI = % of RBCs that are reticulocytes
- 45% = normal hct
- “Days as reticulocyte”: (reticulocyte released this many days early)
- 1 day if hct is 36-45
- 1.5 days if hct is 26-35
- 2 days if hct is 16-25
- 2.5 days if hct is <15
What should the RI be for a person without anemia?
What will it be in a person with anemia?
- normal = between 0.5%- 2%
- anemia = >2%
How does the total iron binding capacity compare between Iron deficiency and anemia of chronic disease?
(Chart)
Serum Iron
Transferrin
Transferrin saturation

What are the other hemoglobin related disorders?
- Hgb with increased O2 affinity
- Hgb with decreased O2 affinity
- Methemoglobinemia- lower affinity for O2, but this increases the affinity of the O2 to the other three hemes, resulting in decreased O2 delivery
- Polycythemia
- polycythemia vera- stem cell disorder
- Secondary Polycythemia due to hypoxia- secondary to cardiac or pulm problem
- Secondary Polycythemia due to increased EPO
What is the Fe cycle?
- Fe picked up from GI and put into bloodstream; associated with its plasma carrier, transferrin
- Delivered to erythrocytes in bone marrow where it is incorporated into hemoglobin
- Mature erythrocytes circulate for 100-120 days before being removed by macrophages
- Macrophages broken down and Fe is returned to bloodstream
- will either go for storage in liver or spleen or back to bone marrow

What is aplastic anemia?
- Not as many hematopoietic cells in bone marrow and they are replaced by fat cells
- Causes pancytopenia- deficiency in all blood cells
What is myelofibrosis?
- Scarring of bone marrow inhibits blood cell production leading to pancytopenia
- leads to blood being produced outside of bone marrow (extramedullary hematopoiesis) which can cause hepatosplenomegaly
- *because there is so much fibrosis, the blood cells sometimes grow in a tight/cramped space and will be wierd shaped?

What are the hemolytic anemias with membrane defects?
- Hereditary Spherocytosis- missing proteins spectrin and ankyrin that hold the RBCs into the innertube shape. Without them, RBCs are round and can’t make it through the obstacle course of the spleen, so they must die.
- Hereditary Elliptocytosis- missing proteins spectrin or glycophorin, making them elliptical or rodlike; also cant make it through spleen
- Acanthocytosis- lack B-lipoprotein causing cholesterol to accumulate on outside making it spiky
- Paroxysmal Nocturnal Hemoglobinuria (not genetic)- lack a protein that protects RBCs and WBCs from complement mediated lysis

Spherocytosis:
genetics
statistics
Whats the problem?
- Inherited in an autosomal dominant pattern
- Most common inherited hemolytic anemia in Europe and US
- frequency of 1 in 5000
- Deficiency of membrane skeletal proteins, usually spectrin and ankyrin
- Episodes of hemolytic crisis can be precipitated by viral or bacterial infection

What are the genetics of elliptocytosis?
Where is it prevalent?
Stats?
- inherited as an autosomal dominant disorder
- Prevalent in regions where malaria is endemic
- incidence may reach 3 in 100 ppl
Acanthocytosis is autosomal ________
recessive

What is paroxysmal Nocturnal hemoglobinuria?
genetics?
Other complications?
What is thought to cause hemolysis in these pts?
- A stem cell disorder which causes complement activated stem cell hemolysis
- patients pass dark urine in morning b/c of hemosiderin in urine
- Acquired disorder (book says it is a mutation in PICA gene located on X chromosome?)
- Patients are at risk of other complications of Hgb release:
- smooth muscle dystonia
- pulmonary hypertension
- renal insufficiency
- hypercoagulability
- thromboses in 40% of pts
- 1/3 develop aplastic anemia
- Thought to be caused by carbon dioxide retentio
Glucose-6-phosphate dehydrogenase deficiency:
What type of anemia is it?
- An anemia caused by a metabolic defect that predisposes RBC breakdown
- G6PD is an enzyme required to maintain the appropriate amount of glutathione in cells to prevent oxidative damage
- other cells besides RBCs will have same deficiency, but RBCs will get hit the hardest since they carry the O2
- After the cells become oxidated (probably by a drug), splenic macrophages will take a bite out of them

What are the types of Metabolic defects that cause anemia?
- Glucose-6-phosphate dehydrogenase deficiency
- Glutathione reductase deficiency
- Pyruvate kinase deficiency
- Methemoglobin reductase pathway (causes shift to right)
- Luebering-Rapoport pathway (causes shift to left)

What is the incidence of glucose-6-phosphate dehydrogenase deficiency?
- X-linked, mostly affects males
- 80% male, 20% female
- 400 million ppl worldwide
- # 1 hemolytic anemia- it is pretty mild, so a good one to have
- fava beans trigger RBC breakdown









