amines, amino acids, polymers/ esters etc ... Flashcards
suggest a use for this
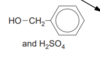
perfume ( remember: these are AROMATIC for a reason )
what type of reaction is it when an alchohol turns into a carboxylic acid ?
oxidation
amino acid group

how do we know compound E is an aldehyde

BECAUSE IT SAYS OXIDISED BY TOLLENS SO ITS AN ALDEHYDE
steps to solving
1. add obvious groups : you know that its an an amino acid so add NH2, and the aldehyde group because it hints that in the question
2. draw it out
whole question answer (WATCH)
https://www.youtube.com/watch?v=-mLdH0F2gOU&t=3s
how else can amino acids be orientated?
like this :

what type of reaction is it when a functional group turns into a alchohol group
hydrolysis

https: //www.youtube.com/watch?v=-mLdH0F2gOU&t=3s
12: 42
1. figure out mass of monomer, then deduct 129 from it to find ou thtat theres a difference of 1* ( mr of H2O ) suggesting that the water was removed

amide group
CONH2

Ester/ amide group

DEFINE DIPEPTIDE

AND DRAW DIPEPTIDES BELOW
FORMS CN bond, C
(note: for this question, just swap the “R” groups around to get both versions of dipeptides )
you cant polymerise the carboxyl and alchoohl group, since they are asking for dipeptides (CN bond polymer)

what would you draw for an ion at its iso electric point (PH)
and what about alkali PH ?
The regular zwitter ion
zwitterion but add OH- to it so that the NH3+ part turns into NH2 . the COO- stays hte same

https: //www.youtube.com/watch?v=KkXJNaRt5-o&t=223s
6: 22
remember: go for the most obvious one, ignore NH2 as in prooton nmr most of the hdyrogens have to be bodned to some sort of carbon

why is it important that single stereoisomers are used in the synthesis of drugs ?
no/fewer side effects increases the (pharmacological)
activity/effectiveness Reduces/stops the need
for/cost/difficulty in separating stereoisomers/optical isomers
REMEMBER: optical isomers, like bindign to taste buds and producin different tastes… can also cause side egects
explain how GC allows us to identify compounds ? (2)
.seperate compounds
. identify compounds by comapring with database
markscheme says “ ignore retention times”
process by which tlc seperates amino acids
adsorption
salt formed when HCL is added to NH2 group in acid hydrolysis ?
NH3(+) CL(-)
dont forget the CL-


