8 - Tissue and Healing Flashcards
Define repair? What are the two types?
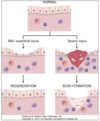
Repair: restoration fo tissue architecture and function after injury
- Regeneration: prolif of residual cells (uninjured) and maturation of tissue stem cells. Basement membrane intact –no scar
-
Healing with scar formation: occurs when restitution is not possible b/c supporting structure severely damaged and/or injured tissues incapable of dividing
- collagen (fibrosis) provides support
- response to severe/chornic damage to lung, liver, kidney
How do non-dividing cells repair? What are examples of non-dividing tissues?
Repair by connective tissue
Neurons and cardiac myofibers
What cells and components are capable of proliferation/regeneration? What signals are needed?
Remnants of injured tissue, vascular endothelial cells, fibroblasts, and tissue stem cells.
Cells respond to signals from growth factors and ECM
-growth factors from mø, stroma, epithelia stimulate cell division, increase size, and promote survival (influence gene expression)
Describe the mechanism of tissue regeneration in epithelia and skin?
Rapid replacement occurs from residual cells and tissue stem cells.
Which parenchymal organs have more capacity for tissue regeneration and repair? What happens if residual cells cannot proliferate?
More limited prolif. of residual cells in: pancreas, adrenal, thyroid, and lung.
Liver has more regenerative capacity
If residual cells cannot prolif, repair by scarring occurs
What are the two major mechanisms of liver regeneration?
- Hepatocyte proliferation after partial hepatectomy: driven by IL-6 and hepatocyte growth factor (HGF) (10% of liver can regrow)
- Liver regeneration from progenitor cells: when prolif capacity of liver cells is impairs
What is the function of stem cells? What is the benefit of them?
They are able to “self renew” and asymmetrically replicate, which means one of the two daughter cells remains a stem cell.
In labile and stable tissues, these are a source of new cells to replace dead ones.
What are the two types of stem cells?
- Embryonic stem cells: pluripotent stem cells able to differentiate into all tissues
- Adult stem cells: lineage specific cells (exp skin or GI epithelium)
- some adult stem cells are multipotent progenitor cells that are present in several tissues (BM for exp) and retain broad differentiation capabilities like fat, cartiladge, bone, endothelium, and muscle.
What is the extracellular matrix and what are the two basic forms? What is each type made by?
Network surrounding cells.
2 forms:
- Interstitial matrix forms 3D gel, made by fibroblasts
- Basement membrane: highly organized interstitial matrix present around epithelial cells, endothelial cells, and smooth muscle cells. Made by mesenchyme and epithelium.
What is the role of ECM?
- Mechanical support
- Regulate cell proliferation
- Provides scaffold essential for healing without scar
- Storage of growth factors: fibroblast GF, hepatocute GF
- creates “microenvironment”
What are the fibrous structural protein of the ECM?
- Collagen: structural proteins provide tensile strength
- Elastin: forms elastic fibers with fibrillin, allowing recoil

What are the highly hydrated cells that are part of the ECM? What is their function?
Proteoglycans and hyaluronan
- Provide compressability (joints)
- Contain growth factors

What are the adhesive glycoproteins and receptors of the ECM?
Fibronectin: major component of interstitial ECM
Laminin: major component of basement membrane
Adhesion molecules: cell adgesions moleules (CAMs) such as immunoglobulins, cadherins, selectins, and integrins

What are the three steps of scar formation? What cell type is involved in each step?
1. Inflammation
- M1 macrophage clear microbes and necrotic tissues to promote inflammation
- M2 macrophage make growth factors to stim cell prolif
2. Proliferation and Angiogenesis
- Epithelial cells, endothelial, fibroblasts can all proliferate
- granulation of tissue - fibroblasts, connective tissue scattered chronic inflammatory cells
3. Remodeling - reorganization of collagen to produce a scar
How does angiogenesis occur? What are the steps?
From pre-existing vessels
- vasodilation via NO and VEGF
- migration of endothelial cells towards injury (VEGF)
- proliferation or endothelial ceells (VEGF and FGF)
- recruitment of pericutes and smooth muscle (PDGF and TGF-B)
- Notch signaling to regulate sprouting and branching

What role do fibroblasts have in the response to injury?
Migrate to site of injury and proliferation b/c of growth factors secreted by endothelium and inflammatory cells.
Deposite ECM: loose collage at first, then more dense and active (scar)
What growth factors are involved in the migration, proliferation, and deposition of ECM by fibroblasts?
TGF-B - transformating growth factor
PDGF - platelet derived growth factor
FGF - fibroblast growth factor
What four growth factors are involved in repair?
- Vascular endothelial derived growth factor (VEGF)
- Fibroblast growth factors (FGF)
- Platelet-derived growth factors (PDGF)
- Transforming growth factor-B (TGF-B)
What is the source and function of vascular endothelial growth factors (VEGF)?
Source: mesenchymal cells
Functions: induces angiogenesis in injury and tumors by stimulating endothelial cells
What is the source and function of fibroblast growth factors (FGF)?
Source: macrophages, mast cells, endothelial cells, fibroblasts.
Functions: induces angiogenesis; promotes migration of fibroblasts, epithelial cells, and macrophages.
What is the source and function of platelet-derived growth factors (PDGF)?
Source: platelets, macrophages, endothelial cells, smooth muscle cells, and epithlium
Functions: induces fibroblast, smooth muscle, endothelial cell proliferation and migration. Stimulates production of ECM.
What is the source and function of transforming growth factor-B (TGF-B)?
Source: Platelets, endothelium, epithelium, lymphocytes, macrophages, smooth muscle cells, and fibroblasts.
Function: suppresses endothelial prolif./migration and acute inflammation; stimulates production of ECM proteins
What is a “hallmark” of the repair protess in wound healing?
Granulation tissue: fibroblasts (collagen) and endothelial cells proliferate (new vessels)

How do scars remodel over time?
Decreased vessels
Some degredation of collagen and other ECM proteins by matrix metalloproteinases (MMP) containing zinc secreted by fibroblasts, macrophages, and PMNs.

How does the appearance of granulation tissue compaed to that of a mature scar?
Granulation tissue: loose connective tissue and edema with many blood vessels
Mature scar: mature collagen and less vessels
(collagen is in blue)

Describe first intention (“primary union”) wound healing and second intention (“secondary union”) wound healing clinically?
First intention: wound closed by approximation of margins, placement of graft or surgical incision closed with stiches/staples. Applies to acute (within 24 hrs) wounds (before granulation tissue)
Second intention: wound left open and allowed to close by epithelialization, granulation tissue, and wound contraction; commonly used to manage infected wounds.
An uninfected surgical incision is approximated by sutures (healing first intention) describe what happens: immediately, within 24 hrs, by day 3-5, and in weeks.
Immediately: incisional space fills with clotted blood
Within 24 hrs: PMNs appear at margin, beginning of epithelialization
Day 3-5: PMNs replaced by mø, granulation tissues fills incisional space, collagen fibers begin to bridge incision
Weeks: scar - connective tissue without inflammation, decresed vessels

When does healing by second intention occur? How does this differ from a first intention?
When extensive damage leaves a tissue defect (large wounds, abscesses, ulceration)
More intense inflammatory response b/c of large fibrin clot and more necrotic material to be removed
- more granulation tissue to fill defect
- wound contrction by myofibroblasts
- substantial scar formation with thinned epidermis
How do nutrition, metabolic status, and circulatory status influence healing?
- Nutrition: VitC deficiency inhibits collagen synthesis
- Metabolic status: may cause persistance of ulcers and infections (DB with impaired PMN/mø function)
- Circulatory status: poor perfusion or obstructed venous drainage prevents in-flow of needed cells/proteins
How do steroids, infections, and mechanical factors influce healing?
Steroids: inhibit TGFB production and decrease fibrosis (inhibits healing)
Infection: prolongs inflammation and may increase local injury
Mechanical factors: increased local pressure may lead to dehiscence (rupture of incision/closed wound).
How do foreign bodies influence healing?
They prolong inflammation
What are two examples of wound healing complications involved excessive repair?
Keloid: raised scar due to excess collagen (mechanism not completely understood); heritable - incresed risk in african americans
Exuberant granulation: protrudes above surrounding skin and prevents re-epithelialization
Describe the formation of contractures? When can this occur?
Wound healing complication in which excessive contraction results in deformity of wound/surrounding tissue.
Examples: occurs after serious burns
If a person is unable to ambulate, they may get an ulcer due to what? What is this ulcer called?
Local ischemia and mechanical pressure.
Decubitus (pressure) ulcer
What are examples of medical condictions that may cause ulcers on the legs/feet? Why don’t these heal well?
Severe varicose veins and congestive heart failure both cause chronic incresed pressure in the veins of the leg.
Lack of healing due to venous stasis causing poor perfusion/delivery of oxygen.
What is this a picture of?

Keloid: hypertrophic scar caused by excessive formation of collagen
What is Ehlers-Danlos syndrome (EDS)? What are common clincal features?
Genetic defects in collagen synthesis or structure
Common features:
- affected tissue lacks tensile strength
- hyperextensible skin, fragile and easily traumatized
- hypermobile joints/ligaments
- rupture of internal organs (colon/large arteries)
The classic type of Ehlers-Danlos syndrome (EDS) results in deficient production of _______?
Collagen type V
What is marfan symdrome and what are common clinical findings?
Mutation affecting fibrillin, a major component of microfibrils in ECM, abundant in aorta, lens, and ligaments.
- Degeneration of aorta (aneurysm/dilation)
- Dislocated lens
- Abnormal mitral/aortic valces
- Long legs, arms, fingers
- Hyper-extensible joints

What are four pathologic contidionts that result in apoptosis?
- DNA damage: radiation, chemo, hypoxia
- Accumulation of misfolded proteins in ER: (neurodegenerative diseases, T2DM)
- Cell injury in certain infections: particularly viral infections like HIV
- Pathologic atrophy in parenchymal organs after duct obstruction: pancreas
What is cirrhosis? What is the appearance? What are causes?
Scarring of the liver - type of repair
In infections of the liver like chronic hepatitis, hepatocytes often regenerate to form fibrosis and nodules.
Alcoholism, primary biliary cirrhosis, and obesity.
What happens to wound strength when sutures are removed?
Wound strength goes from 70% with sutures, to 10% when they are taken out.
What are the basic steps in wound healing?
- Inflammation and formation of scab/clot
- Granulation tissue (24-48 hrs) and angiogensis (day 3)
- ECM deposition and remodeling at about a month or longer (collagen fibers grow into granulation tissue)
____ infections are associated with apoptosis.
Viral infections often cause apoptosis.
What best describes the scar from C-section a week after closer when the sutures are removed?
Granulation is present and no connective tissue is present


