4 - Tolerance and Autoimmunity Flashcards
(37 cards)
How does the immune system recognize all pathogens? What is inherent to this diversity and what suppresses this?
Lymphocyte antigen receptors (T and B cells) through random DNA rearrangement of receptor gene segments.
Inherent generation of autoreactive lymphocytes - the immune system suppresses these (immunologic tolerance)
What occurs if tolerance of T and B cells to self antigens fails?
Autoimmunity
Why is immunologic tolerance important? What are examples?
Abnormal immune responses to self or non-pathogen antigens can cause disease.
Loss of tolerance to self-autoimmunity
Immune response to environment - allergy
” “ transplant - transplant rejection/graft vs host disease
What is central tolerance?
When immature lymphocyte in the generative lymphoid organs encounter antigen (BM for B cells, Thymus for T cells).
This is not complete: small pop of self reactive lymph. escapre but are usually quiescent.
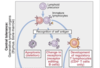
What is peripheral tolerance?
When mature lymphocutes encounter self-antigen in peripheral tissues (lymph nodes, spleen, MALT, peripheral tissues).

What is the mechanism by which central tolerance occurs for B and T cells? What is the exception to this mechanism?
In thymus/BM, immature B/T cells that recognize high avidity self-antigens are apoptosed
Exception: T regulatory cells are self-reactive CD4 T cells that suppress immune responses and express Foxp3
B cells can edit receptors to make new ones instead of being apoptosed.
How does the B cell edit its receptor?
The light chain of the immunoglobulin locus can rearrange and form a new antibody
What is peripheral tolerance and what three things can occur to make sure you don’t react?
When self-reactive T/B cells encounter self antigen in the periphery.
1. Apoptosis:
- Fas/Fas ligand
- Cytokine withdrawl
- AICD
- Anergy: functional inactivation of T and B lymphocytes
- Suppression: T reg cells suppress T cells
What is Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECEP)? What is the classic triad and the underlying defect?
Classic triad:
- Autoimmune hypoparathyroidism
- Autoimmune adrenal insuff.
- Candidiasis
Defects in AIRE gene (autoimmune regulatory gene), a TF that induces expression of self-antigens by thymic medullary epithelial cells (this is how the body makes self so that central tolerance can occur)
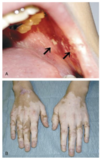
What happens when the AIRE gene is lost?
What is this defect an example of?
- Self-reactive T cells are not deleted
- Self reactive T regulatory cells are not generated
This is an example of failure of central tolerance.
Describe what is necessary for T cell activation?
- TCR on T cell sees antigen on MHC (on APC)
- Co-stimulation: CD28 on naive T cell with B7 (CD80/86) on APC
- B7 upregulated when APC encounters microbe
- When no B7 is present on APC - Anergy
What is Anergy?
Functional inactivation of lymphocytes, occurs when APC lacks co-stim molecule:
- When APC lacks B7 (which normally binds to C28)
- When you have CTLA-4 instead of CD28 (CTLA-4 binds and inactivates B7)
OR PD-1 checkpoint inhibitors, which are induced in T cells and prevent T cell activation

What happens when microbes (pathogen-associated molecular patterns -PAMPs) are recognized by PRRs compared to when self is regognized by PRRs?
They induce B7 molecules on APC surface so APC can activate T cell; this is a danger signal that promots an immune response.
Self antigens don’t stimulate APCs (no PAMPs), they promote anergy.
T and B cells expand rapidly during an immune response and must die after the response is over, what are the mechanisms by which this can occur?
- Activation-induced cell death: repeated activation of mature T cells by self-antigen triggers apoptosis of T cell (and thus removal of self-reactive lymphocytes)
- Fas/Fas Ligand: CD4+ activation ^ expression of Fas “Death receptor” and it’s ligand FasL. Fas:FasL binding causes apoptosis.
- Cytokine withdrawl: Activated T cells (TCR:CD28) express IL2 which allows survival and growth, when stimulus is gone, IL2 isn’t make and there is apoptosis.
What is autoimmuen lymphoproliferative syndrome (ALPS)? What are the clinical characteristics?
Mutations in Fas or FasL - immuen cells dont die properly.
Causes widespread lymphadenopathy, splenomegaly, and autoimmune cytopenias.
Failure of peripheral tolerance
What role do T regulatory cells play in central tolerance?
Express TF Foxp3 - activated in the periphery by self antigen and IL-2 to blunt/suppress actication of T cells through contact-depdendent or contact-independent mechanisms.
Generated by self-antigens in thymus (AIRE)
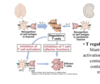
What disease results from a lack of T regulatory cells? What causes this disease?
IPEX: immune dysregulation polyendogrinopathy, enteropathy, Xlinked.
Caused by a mutation in Foxp3 and loss of T regulatory cells. LEads to death if not aggressively treated.
Are tolerogenic self antigens found in generative organs? What about immunogenic foreign antigens?
Tolerogenic self antigens: yes, high concentrations induce negative selection and Treg cellls (central tolerance)
Microbe: no, microbial antigens are concentrated in peripheral lymphoid organs
Describe the persistence of antigen for tolerogenic self antigens vs. immunogenic foreign antigens?
Tolerogenic self antigens: long-liver, prolonged TCR engagement that may induce anergy or apoptosis
Immunogenic foreign antigens: usually short-lived; immune response eliminates antigen
How is HLA/MHC linked to autoimmunity?
The “relative risk” of developing an autoimmune disease is higher in individuals who inherit particular HLA/MHC alleles
- HLA affects which peptides are presented to lymphocytes
Does everyone with a high risk HLA allele get autoimmunity? What is an example of this?
No, lots of people express them but DONT get disease. Complete deficiencies in genes directly involved in the immune response are more likely to cause autoimmunity than high-risk HLA alleles.
HLA-B27 is a strong risk factor for ankylosing spondylitis and about 6% of people are HLA-B27 HOWEVER, the prevelance of AS is only 0.5%
How are environmental factors linked to autoimmunity?
In some cases microbial infection can trigger autoimmunity.
OR, infectious organisms can have antigens that look like self, resulting in autoimmunity (molecular mimicry)
What are the four types hypersensitivity reactions? What is each mediated by?
- Type 1: immediate sensitivity (IgE mediated)
- Type 2: Antibody mediated
- Type 3: Immune complex
- Type 4: delayed type hypersensitivity
What is a type 1 hypersensitivity reaction characterized by? What diseases does this include?
Immediate hypersensitivity: IgE mediated, requires TH2
Diseases: allergic rhinitis, asthma, eczema, and food allergies.



