5.2.2 Respiration Flashcards
(89 cards)
Draw a molecule of ATP, and label it

Draw a molecule of ATP with a Pi

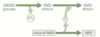
How is ADP and Pi formed from an ATP molecule?
ATP is hydrolysed by ATPase enzyme
what are the 4 main properties of ATP which make is ideal as an energy currency molecule?
High water‐solubility - diffuses rapidly within cells
Hydrolysis releases a useful amount (‘packet’) of energy for a cellular process, but not so much energy that cellular components are damaged
Hydrolysis of ATP (to remove the terminal phosphate group) releases 30kJ mol‐1 of energy
The products of ATP hydrolysis, ADP and Pi, can be re‐used to make new ATP molecules via respiration, either by substrate‐level phosphorylation or by oxidative phosphorylation.
Where does Glycolysis occur?
cytoplasm
where does the link reaction occur?
Mitochondrial matrix
where does the krebs cycle occur?
mitochondrial matrix
where does chemiosmosis occur?
cristae (the folds of the inner mitochondrial membrane)
where does oxidative phosphorylation occur?
cristae (the folds of the inner mitochondrial membrane)
where does the re-oxidation of NADH in anareboic respiration occur?
cytoplasm
What is this? Label it

It is a mitochondion

what is the function of the inner and outer mitochondral matrix (mitochondrial envelope)?
partially permeable barrier - controlls what enters and leave the cell.
what is the function of the inter-membrane space of a mitochondria, and what is it?
the space in‐between the two mitochondrial membranes
The area where hydrogen ions (protons) accumulate - creates steep proton gradient between the mitochondrial matrix and the intermebrane space
inner mitochondrial membrane is not permeable to H+ ions means that the gradient is maintained
what are the cristae, and what is its function?
infolded regions of the inner mitochondrial membrane
It is the site of chemiosmosis and oxidative phosphorylation
cristae provide increased surface area for attachment of Electron Transfer Chain components and ATP synthase enzymes, increasing ATP production
ATP synthase enzyme is embedded in theinner mitochondrial membrane
what is the inner mitochondrial membrane and ATP synthase enzymes reffered as?
stalked particles as the ATP synthases are embedded in the inner mitochondrial membrane, but part of their structure projects out into the matrix
what is the mitochondiral matrix and what does it contain?
the fluid that fills the mitochondrion
Contains:
small circular DNA molecule (mitochnodrial DNA) - contains genes that encode some of the proteins needed in the mitochondrion
18nm (70S) ribosomes - synthesise new proteins
enzymes which are used in link reaction and krebs cycle
what is glycolysis?
A multi‐step metabolic pathway which oxidises glucose to pyruvate
What are the products of glycolysis?
4x ATP (net production of 2 ATP)
2x NADH
2x Pyruvate
What are the named steps of glycolysis?
- Phosphorylation
- Lysis
- Phosphorylation
- dehydrogenation/oxidation
Draw out the steps of glycolysis

Describe the 5 steps of glycolysis
- A glucose molecule (which contains six carbon atoms) is phosphorylated twice, to give hexose bisphosphate; this step involves the transfer of a phosphate group from each of two ATP molecules, i.e. ATP is actually being used, not generated, at the start of glycolysis
- Hexose bisphosphate is split (lysed) into two molecules of the three carbon sugar, triose phosphate
- Each triose phosphate combines with an inorganic phosphate ion from the cytoplasm, to form triose bisphosphate
- Both triose bisphosphate molecules are oxidised to pyruvate; this oxidation reaction requires the coenzyme oxidised NAD, which itself becomes reduced as a hydrogen atom is transferred to it; two reduced NAD molecules are generated in total (one per triose bisphosphate)
- During the oxidation of triose bisphosphate to pyruvate, each triose bisphosphate molecule can pass phosphate groups to two ADP molecules; this step therefore produces a total of four ATP molecules via substrate‐level phosphorylation (transfer of a phosphate group to ADP from a phosphorylated organic molecule).
how many ATP molecules are produced in glycolysis per glucose? therfore what is the total net yield for both glucose?
Per glucose molecule, two ATP molecules are used but then four are produced
OVERALL NET YIELD OF 2 ATP IN GLYCOLYSIS
in aerobic respiration, what happens to the NADH after glycolysis?
the reduced NAD generated by glycolysis enters the mitochondria and drives further ATP production by oxidative phosphorylation.
where does the pyruvate go after glycolysis in aerobic coonditions?
It enters the mitochondria (using specific carrier proteins to cross the outer and inner mitochondrial membranes) and then undergoes the Link Reaction in the mitochondrial matrix.