2.1.2 Biological molecules Flashcards
what is water comprised off, what is its formula and ratio?
Oxygen and Hydrogen
H2O
2 H : 1 O
how would you descrive the distribution of charge in a water moelcule
an uneven distribution of charge between delta -ve oxygen and delta +ve hydrogen
what is the sign for delta?
𝛿
why is the charge described as uneven?
it is due to the pairs of electrons in each of the oxygen-hydrogen covalent bnds is not equally shared, as the electrons are more stronhly attraced to the oxygen atonms nucleaus than the hydrogen atoms nucleas
Polar definition
Molecules that have an uneven distribution of charge are reffered to as polar
Draw a diagram of a water moeclule, showing the uneven distribution of charge

what bond is present IN water molecules
Covalent bonds
what bond is present BETWEEN water molecules
Hydrogen bonds
why are water moleules attracted to each other?
The delta -ve oxygen from one water moelucle will be attracted to a delta +ve hydorgen from another water moelcule
other than water, what other polar groups are there?
Hydroxyl, amine and carbonyl
draw a fiagram of several water moleules, showing the partial charges and the hydrogen bonds
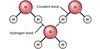
are polar moleucles soulbule or insoaluble and why?
Soluble, becuase water molecules will be attracted to, and will for hydrogen bonds with their polar groups
what is cohesion
when a hydrogen bond forms between to water molecules
what is adhesion
when a water moelcules and a different type of polar molecules for hydrogen bonds
can hydrogen form between two polar moleucles when neither are water?
yes, the same exact thing happens, and example would be two celluose molecules
what is the biological word for polar and water-soluble moleulces?
hydrophilic
hydrophilic deffinition
the physical properties of a molecules which attracts water molecules
are non-polar moelcuels soluble in water and why?
no, they are described as hydrophobic measning they repel water, this is due to the fact they have an even distribution of charge across the moelcules, so they cannot form partial chrages, therfore cannot form hydrogen bonds with water. An example of this is lipids
There are 8 main properties of water you must know what are they?
excellent solvent
effective thermal buffer
high latent heat of vaporisation
low density of ice compared to liquid water
choesion between water molecules
high surface tension
incompressibe
transparent
why is transparancy an important property of water?
it allows light water to pass through water, however its ability to do so depends on the wavelength of light.
this is especially important in aquatic plants and other photoynthetic oganisms which rely on ligh energy to drive photosynthesis (which produces glucose)
why is it good for water to be incompressible?
as water is a liquid, it is incompressible meaning it cannot be forced to decrease in volume.
this is important in insects, which have hydrostatic skeleton to supoort their body.
in plants, cell become turgid when the voume of water in the cell cretes pressure exerted on the cell wall, this is called cell turgor which is vital in enabling the leaves of a plant to be held up to intercept sunlight.
why is it good for water to have high surface tension?
hydrogen bonding from the surface wawter molecules to teh water moleucles below them cretes high surcace tenion, and the water on the top acts a a ‘skin’
this is important becuse small animals such as pnd skaters can move across the surface without breaking through.
why is it good for water to have cohesion between molecules?
water mnolcecules stick togetrher wia hyfrogen bonding. this allows waterto for strong, continuous colums withing a vessel/tube.
These colums have high tensile strength due to the high number of hydrogen bonds between the molecules.
liquids within a vessel will flow from one region to another due to gravity or a hydrostatic pressure difference via mass flow
Effecttive transport mediumn
why is it good that ice is less dense than water
this is due to the fact that water molecules forms a regular semi crystaline structure, with stable intermolecular hydrogen bonds, with the moleucles held far apart from each other.
as ice is less dense than water, ice floats on the surface of water.
Ice on the surace of water insulates the water below, reducing the chances the lower regions freeze. Therefore most aquatic organsism can surviuve even if the upper layers freeze, hence this property creates a stable environment for many species.
why is it good water has a high latent heat of vaporisation
at rtp water is a liquid due to the many hydrogen bonds which holds the moelcuesl very close togethre.
As of this lots of energy input is needed in order to xcayse liquid water to change to a gas state.
This is due to the numerous higydrogen bonds which need to be broken
This means when water evaporates it is an effective cooling method since it will take lots of energy away from the body when it turns to a gas. Hence water is acting as a coolant
why is it good for water be an effective thermal buffer?
water has a high specific heat capacity, meanign it takes lots of energy to increase by 1 degree.
this is due to the numerous hydrogen bonds requiring lots of energy to break.
This means that the temperature of water is very stable.
This is important in cells, since enzymes have a narrow optimum temperature range, and in ponds/lakes where there is a thermostable habitat that doesnt fluctuate.
why is it good water is an excellent solvent
water moelcules are polar, ie there is an unven distrivution of charge between the delta -ve oxygen and delta +ve hydrogen. As of this water molecules are able to form hydrogen bonds with otherpolar molecules and can interact with ions.
Water mpolecules are strongly attracted to a complety surround the moelcecule or ion of a soluble ions so they become dispersed and become dissolved.
This is good as molecules and ions can dissloved in things such as blood plasma for transportation
dilution of toxic substances such an ammonia
what is a condensation reaction?
a reaction is which two monomers are joinded together, forming a new bond, and a molecules of water is released during the reaction
what is a hydrolysis reaction
a reaction is which a molecule of water is used to break a bond.
are condesnation and hydrolysis reactions enzyme-catalysed?
yes
what is an enzyme which catalyses a condensation reaction called?
there is not a general name for these enzymes.
what is an enzyme which catalyses a hydrolysis reaction called?
hydrolytic enzyme
what elements are present in carbohydratres?
C, H and O
what elements are present in lipids?
C, H and O (Phospholipids contain P)
what elements are present in proteins?
C, H, O, N and S
what elements are present in nucleic acids?
C, H, O, N and P
what is a carbohydratre?
Biological moleuces whcih contain only the elements carbon, hydrogen, and oxygen, where there are twice as many hydrogen atoms than oxygen atoms
what is the carbohydrate general formula?
CX(H2O)Y
What is the simplest form of a carbohydrate called? and give 2 example
Monosaccharide
fructose
glucose
what is it called when two monosaccharides are bonded together in a condensation reaction? and what is the bond between the molecules called?
disaccharide is formed
glycosidic bond
what is the bond in carbohydrates called?
Glycosidic bond
what are three or more monosacchardies bonded together called?
polysaccharide
they are bonded into a long chain via glysocidic bonds
what is a hexose sugar?
a sugar with 6 carbons
what type of sugar is glucose
a hexose sugar
what is the molecular formula for glucose
C6H12O6
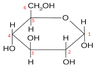
What are the two types of glucose and how do you remember where the hydroxyl (-OH) group is on the carbon 1?
α-glucose = Below
β-glucose = Above
Alpha
Below
Beta
Above
what is the name of the structual variants of molecules?
isomers
Draw α-glucose
draw β-glucose
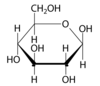
what are the properties of glucose? and its main role?
they contain a polar hyroxyl group (-OH), meaning the can dissolve in water and form hydrogen bonds.
thhis is good so it can dissolve in blood plasma and be transported around the body
its main role is as an energy source is areobic respirtaion where is is broken down and oxidised to release energy
What is a pentose sugar?
a sugar with 5 carbons
what is ribose and can you draw a picture?
a 5 carbon sugar (pentose). Ribose is part of the structure of an rna nucleotide andd part of the strucutre of an atp molecule
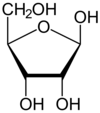
what is deoxyribose and can you draw a picture?
a 5 carbon sugar (pentose). Deoxyribose is part of the strucure of a DNA nucleotide.

what is the difference between ribose and deoxyribose?
deoxyribose has one less oxygen atom than ribose on the carbon 2 of the pentose sugar.
- what is a glycodidic bond?
- When is it formed?
- What is its side product?
- where does it occur in a carbohydrate?
- how are disaccharide and polysacharides formed?
- a covalent bond between two monosaccharides.
- formed during a condensation reaction.
- A water molecule is formed as a side product. This is becuase two hydrogen atoms and one oxygen atom is removed from the monmosacharides, which join together to form water.
- between a hydroxyl from one moonosaccharide and a hydroxyl group from another monosaccharide
- they are formed by the joining of two or more monocsacchardies via glycosidic bonds
what are the two monosacharide in maltose, and can you draw it with labels? and what is the bond called?
2 x α-glucose
α-1,4-glycosidic bond
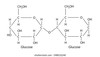
what is sucrose?,what are the two monosacharide in sucrose, and can you draw it with labels? and what is the bond called?
a disaccharide consisting of 1 α-glucose and 1 β-fructose
α,β-1,2-glycosidic bond

what is lactose?,what are the two monosacharide in lactose, and can you draw it with labels? and what is the bond called?
a disaccharide consiting of β‐galactose + β(or α)–glucose, with the second sugar staggered or flipped over compared to the first
in the case of: β-1,4-glycosidic bond

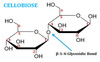
what is cellobiose?,what are the two monosacharide in cellobiose, and can you draw it with labels? and what is the bond called?
a disaccharide consiting of either 2 x β-glucose, where the seconda sugar is flipped over by 180 degrees
the bond is β-1,4-glycosidic bond
what is needed when breaking a glycosidic bond?
water, specifically the atoms of a water moelecules which a reequired to reform the two hydorxyl groups which has previously undergone condensation to form the glycosidic bond.
What are three examples of polysaccharides you must know?
Starch
Glycogen
Cellulose
what is a lipid?
a non-polar hydrophobic compound, mostly made up of carbon and hydrogen atoms, with small proportions of oxygen.
are lipids water soluble? if no, what do you think they are soluble in?
no they are not soluble in water, this is becuase the distribution of charge across the lipid is very even, so cant form partial charges, meaning no hydrogen bonds can form.
HOWEVER
lipiids are soluble in organic solventss such as benzene, as its also non-polar.
what is the difference between a fat and an oil? plus give examples
fat = a lipid which is solid at room temperature, such assubcutaneous fats stored under the skin
oil = a lipid which is a liquid at room temperature, such as oil stored in sunflower seeds
are lipids polymers?
NO! as they do not consist of a chain of specific repeating monomer subunits.
However
They are usually very large and complex moleucles (They are called Macromolecules)
what is a fatty acid?
a carboxyl group (-COOH) attached to a hydrocarbon chain
They are a type of carboxylic acid
Saturated fatty acids description, and where are they present
Saturated = all carbon atoms in the chain form the maximum bnumber of bonds with hydrogen atoms (NO cabon=carbon double bonds)
Straight in shape therefore NO kinks
Animals lipids often contain saturated fatty acids, this is becuause the hydrocarbon chains are straight, saturated fatty acids can pack closely together, resulting in lipids which are solids at rtp
why are solid fats saturated?
Animals lipids often contain saturated fatty acids, this is becuause the hydrocarbon chains are straight, saturated fatty acids can pack closely together, resulting in lipids which are solids at rtp
draw a saturated fatty acid

what is an unsatuirated fatty acids? and what are the two types? kinks?
a fatty acid that contains at least one carbon-carbon double bond.
Monounsdaturated fatty acid = one carbon-carbon double bond, which has only one kink
Polyunsaturasted fatty acid = 2 or more carbon-carbon double bonds, with more than one kink (these are typically found in plants)
The kinks prevent the fatty acid chains from packing tightly together, therefore resuklting in a lipid that is a liquid at room temperature.
how to work out if a lipid is saturated or unsaturated
excluding the -COOH, a saturated fatty acid will have double the number of ( H + 1)compared to C atoms.
For eacgh c=c double bond present, 2 H atoms are lost
what is glycerol?
an alcohol, ie it contain a hydorxyl (-OH)
each hydroxyl group reacts in a condensation reaction with the hydroxyl from the fatty acid chain, therfore this reaction occurs threee time unless its a phospholipid then it occurs twice
what is a triglyceride, what is the bond, and what is its condensation reaction called ?
1 x glycerol + 3x fatty acids
3 x ester bonds
esterification
how is an ester bond broken? what are the enzymes which catalyse this reaction?
hydrolysis by adding water to break the ester bonds
catalysed by lipase enzymes
draw a triglyceride with labels

Give 5 properties of lipids
- Long term energy storage - due to its being very compacts and conmtains many energy rich C-H and C-C bonds, so lots of energy can be sotred in a small place
- Thermal insulation
- Flotation
- cushioning of internal organs
- insoluble in water - doesnt effect the osmotic balcance/ water potential in cells.
what is a phospholipid?
similar to triglyceride, but instead of 3 x fatty acids, there is 2x fatty acid and 1x phosphate group
can you draw a phospholipid?

what is different about a phospholipid than a triglyceride?
part of the phospholpid, the phosphate group is negatively cahrge, meainng it is water soluble and can for hydrogen bonds with water. this is described as a hydrophilic head (includes glycerol and phospahte group)
HOWEVER
the two fatty acids chains dont cahnge therefore they are non-polar and cant dissolve on in water, creating hydrophic tails, so they repel water molecules
what occurs due to the hydrophilic head and the hydrophobic tails?
A bilayer can form when placed in water.
there are two layers of phospholipds, where the fatty acid tails point inwards creating a hydrophobic core free of water, whereas the hydrophilic phosphate heads lie outwards, where they can interact with water.
what is the basis of all biologuical membranes and why?
the phospholipid bilayer, becuase it is partially permerbale and only allows small and lipid soluble molecules free, whereas polar and ions are unable to passs through
give a use of muliple layer of membrane
electrical insulatuion, ie in the myelin sheath around the axon of a neurone
what is a sterol? structure?
steroid alcohols.
very complex structure based on four fused carbon rings
they have ahydrophilic hydroxyl group, howver the rest of the molecules is hydrophobic, meainng their porperties are similar to phospholipids
give an example of a sterol, and state its properties
Cholesterol - a principle sterol in our bodies
- Its a constituent of the plasma membra, wher is fits between the phospholipds, its job is to regulate the membranse fluidity
- Precursor in the synthesis of vitamin D
- A precursor in the synthesis of steroid hormones, suihc as testosterone.
draw me and label a general amino acid

what does the R stand for?
a variable group, as there aer over 20 different amino acids, it is easy just to put R as it can b e interchangable for each amino acid
They vary is size and chemical properties, and may even contain different elements.
what in the central carbon in an amino acid called?
alpha carbon
how many different amino acids are there?
20
what is the only amino acid containing a sulfur atom?
Cysteine, its R group is -RH, therfore is can form disulfide bonds.
what is the difference between essential and non-essential amino acids?
essential amino acids- they cannot be made in the body, and can only be obtained via our diet
non-essential amino acids- these can be made in out body from essential amino acids, in a process called transamination.
what is the bond in polypeptides called?
peptide bond
how does a peptide bond form? what is the reaction called? bi-products?
a reaction between the amine of one amino acid and the carboxyl from another amino acid
condensation reaction
water
draw a simple dipeptide and clearly label the peptide bond

how are amino acids assembled in the cells?
ribosomes join together thae amino acids, into a specific sequence determined by the mRNA, in a process called translation.
what enzyme catalyses translation?
peptidyl transferase.
what is the name of many amino acids joined together with peptide bonds when forming a long unbranched chain?
A polypeptide
what is a protein
One or more polypeptides which have a specific shape and biological function
how do you calculate the number of different polypeptides?
20n
i.e pentapeptide = 205 = 3.2 x 106
what is the name of the enzymes which catalyses polypeptides back into amino acids?
protease enzymes
Primary structure of proteins
The specific sequence of amino acids, bonded together by peptide bonds
how is the primary structure of proteins determined?
its determined by the order od DNA bases in the genes which encodes that protein, in short its genetically determined
Secondary structure of proteins
chain coil - alpha helix
fold back - beta pleated sheets.
The only bonding present here is intramolecular hydrogen bonding
(between delta -ve oxygen and delta +ve hjydrogen)
DONT FORGET
some regions may not adopt either of these strucure but instead form a random coil.
how is the secondary structure of proteins determined
its the result of further folding and hydrogen bonding at regions along the amino acid sequence
Teritiary structure of proteins
Hydrophobic interactions - the intercation between non-polar R groups interacting with only one another, and excluding water and other polar R groups. ie creating a hydrophobic core inglobular proteins
Hydrophilic interactions - water soluble R groups will be attracted to watcer and to eaxh other, leading to the formation of hydrogen bonds between delta +ve and delta -ve atoms. ie creating the hydrophilic surface in globular proteins. Creates good solubility
Disulfide bonds - only occur when there are two sulfur atoms present in the R groups, which can only happend if two cysteine amino acids are present in different region of the polypeptide chains. (Only seen in proteins secreted out of the cell)
ionic bonding - these are the stong electrosation attractions between a positive and negative ion. Normallu occurs, when one R group loses a hydrogen ion, and another R group gain a hydrogen ion. if pH is changed, the shift in hydrogen ions can negatively affect the ionic bonding.
how is the teritary structure of proteins determined?
by futher folding of the polypeptides into its 3D shape. This involves various types of bondings between tbe R groups of amino acids in the polypeptide chain.
these being: hydrophobic interaction, hydrophilic interactions, disulphide bonds, ionic bonds and hydrogen bonds.
Quaternary structure of proteins
only some proteins
same bonding as the tertary strucute
Occurs when more than 1 polypeptide chains joined together to form the final 3D shape.
what is a globular protein?
proteins in which the polypeptide chain(s) re folded into a compact, roughly spherical.
The hydorphobic R groups are burried in the core of the proteins, hence the name hydrophic core, and the hydrophilic R groups are mostly exposed on the surface of the protein, therefore globular proteins have good solubility in water.
many of them are conjugated, meaning they have a permenantly attached prosthetic group that is essentialy to carry out their function.
are globular proteins soluble?
The hydorphobic R groups are burried in the core of the proteins, hence the name hydrophic core, and the hydrophilic R groups are mostly exposed on the surface of the protein, therefore globular proteins have good solubility in water.
what are 3 examples of globular proteins?
- Insulin
- Catalase
- Haemoglobin
Describe the function, structure and properties of INSULIN
Conjugated Protein
Function: A hromone which control the blood glucose levels via cell signalling. insulin binds to specific complementary receptors on the plasma membrane of the lvier and muscle cells triggering them to absobed the excess glucose
Structure: Insulins tertiary structure has a very specific 3D strucutre, therefore the polypeptide chain must fold the same way for every single insulin protein, this is important as inslin is complementary and wouldnt be able to work if its hsape want corrrect.
Insluin has good solubility which is achieved by the hydrophilic R group surface of the globular protein
Describe the function, structure and properties of CATALASE
CONJUGATED PROTEIN
Function: an enzyme which acts as a biological catalyst in the process of breaking down hydrogen peroxide (a toxic waste product of cellular metabolism) into oxygen and water
structure: there is more than one polypeptide in the quaternary structure, also it is a conjugated protein meaning it has a permentant prothetic group attached. In this case the prothetic groups are haem groups, which contain fe2+ ions.
Describe the function, structure and properties of HAEMOGLOBIN
Found in erythrocytes (red blood cells), whwerew is role is to reversibly combine with oxygen and hence carry oxygen in the blood from he lungs to repireing tissue.
structure: there are 4 polypeptide chains prsent in the quaternary structure, thse being 2x alpha-globin and 2x beta-globin
properties: high solubility do itr can redily dissolve in the cytoplasm of erytherocytes, which is achieved by the hydrophilic R group surface of the globular protein
conjugated: like catalase, it has prothetic groups of haem, each polypeptide chain has one heam group each, therefore there are 4 haem groups, an there is an Fe2+ ion in each haem group.
one molecule of oxygen can bind to one haem group therfore one haemoglobin molecule can carry up to 4 oxygen molecules each.
what is a fibrous protein?
elongated proteins, where the polypeptide chains do not fold up into a sperical shape.
they tend to be very long and insoluble, due to their massive size and presence of many amino acids with hydrophobic R groupos exposed to the surface.
High tensile strength, due to the forming of long ‘rope-like structure’
Metabolically unreactive
Flexible
less variety in amino acid types in their primary strucutre, and thier primary structure is quite repetative.
properties and functions of collagen
Fibrous protein
very high tensile strength, insoluble but flexible
found in the connective tissue of skin, ligaments and tendons
high tensile strength due to the strucutre, where three polypeptides wound around one another in a rop like strucure, also there are numerous molecules crossed lonked with many hydrogen bonds.
properties and function of keratin
fibrous protein
insoluble, high tensile strength, relatively inflexible
present in hair and nails
contains a very high proportion of sulfur containg cystein in the primary structure, this allows for numerus disulfide bonds in the tertiary strucure, giving incresed stregth, but reduced flexiility in the protein.
properties and functions of elastin
fibrous protein
stretchy (allows for the walls of the alveloi and artieries to expand and passively recoil to return to their orginal shape.
found in elastic fibres
Quaternary strucutre made up of many different TROPELASTIN POLYPEPTIDES cross linked to one another.
test for proteins
Biuret reagent
sodium hydroxide solution mixed with copper sulfate solution
add it to the sample, if there is a colour change from pale blue to lilac/purple colour
test for starch
add a few drops of iodine solution to the test sample, if the colour chanfges from yellow to blue/black, starch is present
test for lipids
the sample should be mixed with equal volume of ethanol and water and shake, if lipids are present a milky white emulsion should form
test for reducing sugars
Benedicts test ( alkalime copper (II) sulfate)
all mono and most disacharides are capable of donating electrons
add an equal volume of benedicts solution to the test sample, and heat at 80 degrees celcus for around 10 mins.
none: pale blue solution
little: green ppt
some: orange ppt
lots: brick red ppt
test for non-reducing sugar
gives negative result to benedicts reagent.
non-reducing sugar can be hydrolysed into reducing sugars components
- boil in dilute HCl
- if before hydrolysation its negative, and then test positive after hydrolysation the conclusion is that a non-reducing sugar was present in the original sample.
reagent test strips for reducing sugars
dipped in sample, and if there is a colour change it can be compared with colour chart giving an approximate concentration of reducing sugars.
properties and strucutre of starch
energy storage polysaccharide
2 differnet alpha glucose polymers, amylose and amylopectin
can be readily hydrolysed by enzymes, releasing alpha glucose
starch is compact, very low solubility therefore doesnt effect osmotic potential.
properties and structue of glycogen
energy storeage polyscacharide in animal and fungal cells.
alpha glucose polymer branched structure
1,4 and 1,6
lots of branching therefor very compact
Some coiling
branches mean quickly hydrolysed fior a quick release of glucose during areobic respiration
insolube, therfore doesnt effect osmotic balance
stored as glycogen granules
properties and strucure of cellulose
beta-glucose
1,4 only
used in plant cell walls
linear, unbrached
each beta glucose is at flipped 180 degrees, creating a perfect straight chain, with no coiling
they bundle togerhter in parallel by many hydroge bonds, forming cellulose microfibrils which have an extremely high tensile strenth whilst remaining flexibile.
insolub;e, meaing they can be used as cell wall, as they ownt be dissolved bu water, its also unreactive so it wont weaken during internal metabolic reactions.
amylopectin in starch properties
aplha glucose
1,4 and 1,6
same orientation
branched
some coiling
low solubility
starch grain
present in plant chloroplast
Energy storage
amylose in starch properties
alpha-glucose
1,4 only
same orientation
linear
coiled
low solubility
starch grain
present in chlorplast
energy store


