4. Amino acids Flashcards
An amino acid is a carbon compound with both what?
An amine group (-NH2) and a carboxyl group (-COOH).
Both of these groups are attached to the same carbon, which we call the central carbon atom. What are the other two groups attached?
A hydrogen atom (H) and a variable amino acid side chain (when unspecified, we use R)

What makes each amino acid unique?
Side chain or R group.
Scientists have discovered up to five hundred naturally occurring amino acids. Out of this number, the human body only uses how many amino acids to make all the proteins that keep us alive?
20
In the amino acid glycine, the side chain is what?
Hydrogen.

Most naturally occurring amino acids are what?
α-amino acids.
An α-amino acid is one where the two functional groups (−NH2 and −COOH) are attached to the same carbon atom. Cells use these amino acids to synthesise proteins.
Amino acids are chiral, meaning that amino acids are capable of forming what?
Enantiomers—mirror image molecules.

Which enantiomer is more common in nature, which cells use to build proteins?
The L form.
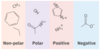
What are the 4 general groups of amino acid?
- Non-polar amino acids have non-polar side chains, typically alkyl groups.
- Polar amino acids contain polar groups such as −OH or −CONH2.
- Positively-charged amino acids typically contain additional −NH2 groups.
- Negatively-charged amino acids typically contain additional −COOH groups.
Amino acids also share some common properties, namely their ability to form what?
Zwitterions.
Amino acids may act as an acid or a base depending on the pH of the solution.
When the solution is acidic, there is an excess of what?
Protons (H+).
Because amino acids can act both as an acid or a base, we call them what?
Amphoteric.
The isoelectric point (pI) is the pH where the amino acid is at a net neutral charge, i.e. where the zwitterionic form is most dominant.
This is not the same as neutral pH (7), because some amino acids contain extra −COOH or −NH2 groups in their side chains, skewing the balance in one direction. If there are more −COOH groups, the amino acid needs more acidic conditions to become fully neutral; if there are more −NH2 groups, the amino acid needs more basic conditions to become fully neutral. As a result, each amino acid has a differentwhat?
Isoelectric point.
We can make use of the isoelectric point in electrophoresis.
Electrophoresis is a procedure that separates molecules based on their movement under the influence of what?
An electric field.
To perform this procedure, scientists insert a solution of mixed molecules in the middle of a medium (typically a gel). They buffer the pH of the medium to a set value, and then apply an electric field.
At one end of the medium lies the anode, a positive electrode which attracts negatively-charged particles; on the other, the cathode, a negative electrode which attracts positively-charged particles.
How does this apply for an amino acid?
When the pH is more acidic than the pI, the amino acid becomes positively charged, so it moves to the cathode.
When the pH is more basic than the pI, the amino acid becomes negatively charged, so it moves to the anode.
Glycine has a pI of 6.1, while cysteine has a pI of 5. Say we buffer the solution to a pH of 5.5.
This is more acidic than 6.1, so glycine will be in the positively-charged acid form.
This is more basic than 5, so cysteine will be in the negatively-charged base form.
Under the influence of the electric field, glycine will move to the _____, while cysteine moves to the _____.
Cathode, anode.

Amino acids link together in chains to make up what in our bodies?
The proteins.
Fortunately, humans can synthesise eleven of the twenty amino acids that make up our proteins. But what about the other nine?
These nine amino acids are termed essential because we cannot synthesise them—they must come from where?
Our diets.

Do branched chain amino acids help you gain muscle?
The multimillion dollar fitness and nutrition industries promote some amino acids over others. They claim that certain “branched chain amino acids” (BCAAs) are especially useful in promoting muscle growth.
No. Just a high protein diet will do it.
Amino acids react together in a condensation reaction to form what?
A peptide.
In a condensation reaction, the carboxyl group (−COOH) of one amino acid reacts with the _____ group (−NH2) of the other amino acid.
Amino.

Glycine and alanine combine together to form a peptide, a chain of amino acids. Because this peptide is only two amino acids long, we call it a what?
Dipeptide.
The dipeptide begins with an amino group and ends on which group?
A carboxyl group.

Peptide chains can be many amino acids long.
As the peptide chain grows longer, the terminology we use also changes.
What is the name of the longest chain?
Protein.

The peptide link is the bond between the carboxyl group and the amino group, abbreviated as −CONH−. As we mentioned before, the positioning of the hydrogen atom next to the C=O bond does not matter.
The easiest way to identify the peptide link is to keep an eye out for nitrogen atoms. Once you find one, look for a neighbouring carbonyl group (C=O). Be sure to circle both the ___ and the ___.
Nitrogen atom and the carbonyl group.

What is the shortest protein, and how many amino acids does it have?
Insulin.
Lack of insulin production can cause diabetes.
What do we call the reaction that breaks apart peptides and proteins?
Hydrolysis.
“Hydro” refers to water, which is necessary for this reaction. Condensation reactions remove water; hydrolysis essentially adds that back in. “Lysis” is Latin and refers to the splitting of the molecule, originating from the Greek “lusis”.
Protein hydrolysis can only occur under what conditions?
Heat and acidic conditions
Say you’ve sunken your teeth into a tofu burger. The protein in that tofu patty doesn’t magically appear in your muscles.
After you’ve chewed your burger into itty-bitty pieces and swallowed, the food travels through your digestive tract and into the stomach. The stomach produces the very same acid that scientists use to help split proteins apart, which is what?
Hydrochloric acid.
The acid and the enzyme pepsin (a type of protein) catalyses the hydrolysis of the proteins in your meal, partially digesting the food.
The food then makes its way to your duodenum (small intestines), where more enzymes (trypsin and chymotrypsin) finish off the digestion. The resulting amino acids are absorbed into the bloodstream, free to be repurposed however your body needs it—perhaps a new muscle protein or an enzyme!


