15. Type 1 Diabetes Mellitus Flashcards
Ambiguous cases of diabetes classification
Type 1 diabetes leading to insulin deficency can present LATE= latent autoimmune diabetes in adults (LADA)
T2DM can present in childhood (Associated with childhood obesity)
Some people with T2DM can present with diabetic ketoacidosis (though this is more common in type 1)
Monogenic (e.g mitochondrial) can be present phenotypically as either type 1 or 2
Can present in pancreatitis
Can follow the following endocrine disorders:
- Phaechromocytoma
- Cushing’s syndrome
- Acromegaly
All cause hyperglycaemia

Aetiology of T1DM
- Normally there is an environmental trigger
- Occurs in a background of a genetic component
- Together this leads to autoimmune destruction of islet cells
- T2DM has a BIGGER genetic component
Clinical use of C-Peptide
Can be measured in the blood as a marker of insulin function since it is linked to insulin production
Significance of loss of first phase insulin
This is an indicator that the patient will develop diabetes later on, though this can take years
How is T1DM a ‘relapsing, remitting disease’
- Over time the beta cells reduce, then stabalise, then reduce again
- This may be due to an imbalance between effector T-cells and T-regulatory cells (effector cells cause destruction of beta cells and T- reg cells control this destruction)
- Over time the effector T cells increase in number and the T-regulatory cells decrease
relevance of autoimmune aspect of T1DM
This leads to increased prevalence of other autoimmune disease such as rheumatoid arthritis, thyroid disease etc.
Coupled with:
- Risk of autoimmunity in relatives
- More complete destruction of beta cells (all will be destroyed in T1DM)
- Could be a novel treatment
Histological features of T1DM:
There will be a large amount of lymphocyte infiltration of the beta cells
Alleles which confer a genetic susceptability to T1DM
- HLA is located on chromosome 6
- HLA-DR1-6 are alleles conferring risk (In particular DR3 and DR4)
Diabetes markers- used to clincially assert the prognosis of T1DM
TWO most significant markers:
- Islet Cell Antibodies (ICA)
- glutamic Acid Decarboxylase Antibodies (GADA)
Other two antibodies: not measured in clinical practice
- insulin antibodies (IAA)
- insulinoma-associated-2 autoantibodies (I1-2A)
Presentation of Type 1 Diabetes: signs and symptoms
Symptoms:
- Polyuria
- nocturia
- polydipsia
- blurring of vision
- ‘thrush’- Increased risk of infections
- weight loss
- fatigue
Signs:
- dehydration
- cachexia
- hyperventilation- may have metabolic acidosis
- smell of ketones
- ketonuria
Other hormones that increase hepatic glucose output
- Catecholamines
- Cortisol
- Glucagon
- growth hormone
Action of insulin
Insulin has a negative effect on:
- hepatic glucose
- protein breakdown in the muscle
- Glycerol being taken out from the fatty tissue into the periphery
Has a positive effect on:
- Glucose being taken up by the muscle
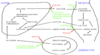
Mechanism of Diabetic Ketoacidosis
- Glucose can no longer be taken up into our cells and utilised so a lot out our energy comes from fatty acids
- Lipid in the adipocytes is broken down
- Normally glycerol is released from the adipocytes but in the case of insulin deficiency you get FATTY ACID RELEASE
- These are then converted to ketones in the liver (A process normally prevented by insulin)

Aims of T1DM treatment
- Reduce early mortality
- Avoid acute metabolic decompensation
- Prevent long term complications:
- retinopathy
- Nephropathy
- Neuropathy
- Vascular Disease
Diet in T1DM
- Reduce calories from FAT
- Reduce calories from REFINED CARBOHYDRATES
- Increase calories from COMPLEX CARBOHYDRATES
- Increase SOLUBLE FIBRE
Balance distribution of food over the course of a day with regular meals and snacks
Classification of insulin treatments
Can be:
1. With meals:
- Short acting insulin
- Human insulin
- Insulin analogues: Lispro, Aspart, Glulisine (mimic physiology)
2. Background insulin:
- Long acting
- Non-C bound to zinc or protamine
- Insulin analogues- Glargine, Detemir, Degludec
insulin Profiles of treatment
- Non- diabetic insulin profile- Trying to mimic normal physiology (rising and falling with gluccose meals)
- Twice daily insulin with intermediate insulin- They can inject themselves with the short-acting insulin after meals. They have the intermediate insulin to mimic the basal insulin level
- Newer analogues- can give basal insulin that lasts longer, given to patients with SEVERE HYPOGLYCAEMIA (lispro or novorapid with long acting glargine or determir)
- Insulin pump- continuous insulin delivery with pre-programmed basal rates and a bolus for meals, does NOT measure blood glucose, risk of acidosis if taken off
Islet cell transplants process
- Islet cells are harvested, isolated and injected into the liver
- There is a risk of rejection so patients must be on immunosuppressants for life
- Islet cells are difficult to get a hold of so there is a very long waiting list
- People who are legible for islet cell transplants have long term type 1 diabetes mellitus with complications and occurrences of severe hyopglycaemia that can’t be controlled by the instant pump
Capillary monitoring of blood glucose levels
Can be measure by pticking the finger:
This is reflective of venous blood glucose
Based on this reading the paitient can titrate their inulin dose
Alternatively a continuous glucose monitor attached to subcutaneous tissue can be used- not as accurate as capillary monitoring
HBA1c measurement
- A method of meausring long-term blood glucose
- HBA1c= glycosylated haemoglobin
- A marker for the last 3 months (though rate of glycation may be faster in some)
- There is a good correlation between plasma glucose over time and HBA1c levels
- We aim for HBA1c<7% in T1DM
- Lower levels are associated with lower risk of microvascular complications
- Diseases of high haem turnover make the reading innacurate
Acute complications of T1DM
- HYPERGLYCAEMIA
resulting from:
- Reduced tissue glucose utilisation
- Increased hepatic glucose production
- METABOLIC ACIDOSIS
- Circulating acetoacetate and hydroxybutyrate
- Results from increased ketone body production in the liver
- Osmotic dehydration and poor tissue diffusion
also can occur in T2DM:
- DKA coomon in black and asian patients with T2DM
- DKA in T2DM can also occue at times of pancreatic insufficiency
Hypoglycaemia in diabetes (‘hypos’)
Hypos are inevitable as a result of treating diabetes:
Definitions:
- Hypoglycaemia= plasma glucose <3.6 mmol/L
- Severe hypoglycaemia= any event that requires another perso to treat it (may contribute to arrhythmia and sudden death
Mental processes are impaired at <3mmol/L
Conciousness impaired at <2mmol/L
Recurrent hypos result in loss of warnings- hypoglycaemia unawareness
- Main risk factor is the quality of glycaemic control
What causes hypoglycaemia?
- Unaccustomed exercise
- missed meals
- Inadequate snacks
- Alcohol (can become unaware of hypoglycaemia)
- Innapropriate insulin regime
Signs and Symptoms of hypoglycaemia
Can be due to increased autonomic activation (1) or impaired CNS function (2)
1.
- Palipitations (tachycardia)
- Tremor
- Sweating
- Pallor/ cold extremities
- Anxiety
2.
- Drowsiness
- Confusion
- focal neuropathy
- Altered behaviour
- coma
Treating hypoglycaemia
Can be treated by oral or paraenteral means
ORAL:
- FEED THE PATIENT
- Glucose- rapidly absorbed as solution or tablets
- Complex carbohydrate- to mauntain blood glucose after initial treatment
PARENTERAL:
- Give if conciousness is impaired
- IV dextrose e.g 10% glucose infusion
- 1mg glucagon IM
- Avoid concentrated solutions


