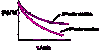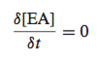Week 1 - enzymes Flashcards
(75 cards)
Describe the common nature of enzymes and what gives rise to the 3D structure of the enzyme
- Enzymes are proteins with catalytic activity
- They are polypeptides
- The polypeptide chain “folds” which gives rise to the 3D structure of enzymes
Describe the active site of the enzyme
- The active site is part of the tertiary structure and is responsible for the catalytic activity of the enzyme
- It is generally a hydrophillic cavity containing amino acids with side chains which are arrange to interact specifically with the substrate in an attractive manner
- The indentation on the surface of the enzyme is complementary in shape to the substrate

Outline the specificity of enzymes
- Enzymes are highly specific both in binding to substrate and catalysing reactions
- Stereospecificity is due to a series of specific non-covalent enzyme-substrate binding interactions
- The chirality of the active site (proteins consist mainly of L-amino acids) ensures that it is able to bind to one enantiomer of substrate
What is the chirality of the active site due to?
- The chirality occurs because the amino acids in the polypeptide are mainly L-amino acids
What are the types of enzyme-substrate interactions used by enzymes?
- There are four types of interactions used by enzymes
- Electrostatic interactions
- Hydrogen bonding
- van der Waals interactions
- Hydrophobic interactions
Name the different classes of enzyme structural types
- Metallo-enzymes
- Membrane-associated enzymes
- Glycosylated enzymes
Describe the metallo-enzyme structural type
- Metallo-enzymes are enzymes that bind metal ions, which typically have a metal cofactor located at the active site of the enzyme

Describe the membrane-associated enzyme structural type
- Membrane-associated enzymes are enzyme associated with biological membranes and consists of two classes:
- Extrinsic membrane proteins which are situated on the surface of the membrane
- Intrinsic (or integral) membrane proteins which are situated within the membrane bilayer

Describe the glycosylated enzyme structural class
- Glycosylated enyzmes arise due to the attachment of carbohydrates to the peptide backbone of the protein
- The attachments of the carbohydrate residues are typically required for full activity of the enzyme rather than participating in active site catalysis
Describe enzyme function
- Once the substrate is bound the enzyme starts to catalyse its specific chemical reaction using active site catalytic groups and releases its product back into solution
- They function to catalyse biochemical reactions
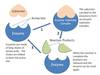
Describe the hallmarks of enzyme catalysis
- Speed - enzymes catalyse reactions several folds faster than uncatalysed reactions
- Selectivity - once the enzyme is bound to its substrate via specific binding interactions at the active site, they are able to recognise subtle changes in substrate structure
- Specificity - enzymes select unique site of action within the substrate and therefore catalyse the reaction with precision
What are cofactors commonly described as?
- The enzymes “chemical teeth”
What enzymes might have cofactors?
- Enzymes that participate in oxidation-reduction reactions and group transfer processes are mediated by cofactors

Gives some examples of cofactors
How may the cofactors be associated with the enzyme?
- Metal ions such as Zn2+
- Organic molecules such as coenzymes - e.g. NAD+ in yeast alcohol dehydrogenase (YADH)
- They are transiently associated with the enzyme, effectively functioning as a co-substrate
What are cofactors called that are permanently associated with their protein?
- Cofactors which are permanently associated with their protein are called prosthetic groups
What happens to a coenzyme during an enzymatic reaction and what must happen afterwards?
- Coenzymes that participate in an enzymatic reaction are chemically changed during the reaction and must be returned to their original state upon completion of the catalytic cycle
How is the chemical change that occurs in a prosthetic group different to that of a coenzyme?
- The chemical change that occurs for a prosthetic group can be returned to its original state in a separate phase of the enzymatic reaction
What are some coenzyme precursors?
- Many vitamins are coenzyme precursors
For the reaction given below, what is the order of the reaction and how do we define the instantaneous rate / velocity?

- The reaction is first order
- K1 is the rate constant for this reaction and the units are s-1
- Rate / velocity (v) is defined in terms of the time (t) dependant production of protein P
- The formation of P involves the loss of A
- v is defined in terms of the time-dependant consumption of A
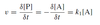
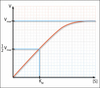
Describe the concentration-time graphs of a first order enzymatic reaction using the examples of A (substrate) and P (product)
- Transformation of substrate to product is unaffected by concentration
- Converstion of A to P results in the concentration of A decreasing exponentially with time
- k1 is directly proportional of [A] so reaction is 1st order
- [A] decreases exponentially with time so plotting ln([A]) versus t produces the straight line which is characteristic of a 1st order reaction

What is one of the characteristics of a 1st order reaction?
- The half life (the time taken for A to decompose) is constant and independant from the initial [A]
Write the equation for a second order reaction and explain why it is considered to be second order
- When two product react together to form a product the reaction is considered to be second order
- The reaction is considered to be second order because the rate is proportional to the second power of the concentration
- The rate of this reaction is proportional to the consumption of A and B and to the formation of P
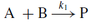
Write the rate equation for a second order reaction using A and B as substrates and P as the product
- The reaction is considered to be second order because the rate is proportional to the second power of the concentration
- The units for this equation are s-1M-1

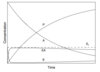
Describe the difference between a first order and a second order concentration time graph
How is the half life for a second order reaction defined?
- The second order reaction graph has a steeper curve compared to the first order curve
- The half life for the second order reaction is defind as t1/2 = 1/(k1[A0]) which is dependant on the initial reactant concentration