W4 Noradrenergic Neurotransmission (Ben) Flashcards
What is the general structure of a catecholamine?
A benzene diol (2 OH grps) with an amine-containing side chain
(img = benzene diol)

From which AA are catecholamines synthesized?
Tyrosine
What is the first step in the synthesis of catecholamines?
Substrate?
Enzyme (and its action)?
Product?
Hydroxylation of benzene ring…

Substrate: Tyrosine + O2
Enzyme: Tyrosine Hydroxylase
Product: DOPA + H20
What is the cofactor for tyrosine hydroxylase?
And what does it become after the reaction?
Tetrahydrobiopterin
- becomes dihydrobiopterin

What is the inhibitor for the first enzyme in the synthesis of catecholamines?
What kind of inhibition is it?
What can it be used to treat?
α-methyl-p-tyrosine inhibits tyrosine hydroxylase
- competitive inhibition
- used to treat inoperable pheochromocytoma, an NE-producing tumor of the adrenal medulla
What is the 2nd step in catecholamine synthesis, after hydroxylation?
Reactants?
Enzyme?
Products?
Decarboxylation of the tyrosine-derived carboxyl group…

Reactants: DOPA
Enzyme: Aromatic AA Decarboxylase
Products: Dopamine + CO2
What is the cofactor for the 2nd enzyme in catecholamine synthesis?
Pyridoxal Phosphate (PLP)
- active form of vitamin B6
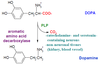
In what tissues is aromatic AA decarboxylase found?
In catecholaminergic AND serotonergic neurons, as well as blood vessel + kidney tissues
What is the 3rd step in catecholamine synthesis, after decarboxylation?
Substrate?
Enzyme?
Products?
Hydroxylation of dopamine’s amine side chain (B position)…

Reactants: Dopamine + O2
Enzyme: Dopamine β-Hydroxylase
Products: Noradrenaline + H2O
What is the co-factor for the B-hydroxylation of dopamine?
Ascorbic Acid
- becomes dehydroascorbate
(via Dopamine B-hydroxylase)

What is the last step in catecholamine synthesis, after the 2nd hydroxylation?
Reactants?
Enzymes?
Products?
Methylation of the amine group…

Reactants: Noradrenaline
Enzyme: Phenyl-ethanolamine N-methyl Transferase
Products: Adrenaline
What is the cofactor for the enzyme which produces adrenaline?
S-Adenosyl Methionine
- methylates noradrenaline and becomes S-Adenosyl Homocysteine

What is the rate-limiting step in catecholamine synthesis?
The first step catalyzed by Tyrosine Hydroxylase…
hydroxylation of the benzene ring of Tyr

Phenyl-ethanolamine N-methyl transferase is present only in which cells?
A cells of the adrenal medulla
and small groups of neurons in the brain stem
(only these cells produce adrenaline)
Name 3 mechanisms for regulation of the enzyme which produces DOPA.
Tyrosine hydroxylase is regulated by:
- Noradrenaline - negative feed-back inhibition
- Ca++ - activates the enzyme
- Kinases - PKA/PKC/Ca-Calmod.-dep. Kinase all phosphorylate + stimulate the enzyme
How does phosphorylation effect tyrosine hydroxylase?
It stimulates it by increasing affinity for its tetrahydrobiopterin co-factor.
How does sympathetic activity affect noradrenaline synthesis?
In the short term?
And long term?
Include enzymes affected + mechanisms.
Short term: It increases tyrosine hydroxylase activity (despite the usual negative feed-back inhibition of the enzyme via noradrenaline).
Ca++ enters the nerve terminals via VDCCs in sympathetic activity, thus activating the enzyme.
Long term: Prolonged sympathetic activity leads to upregulation of de novo synthesis of Tyr hydroxylase and Dopamine B-hydroxylase.
How do steroid hormones affect the synthesis of catecholamines?
by increasing activity of the N-methyl Transferase…
they increase the synthesis of adrenaline
(according to the physio lecture, it is specifically cortisol which does this, when it reaches the adrenal medulla in high concentration as the microcirculation of the zona fasciculata flows towards the medulla)
What altered catecholamine substrate can be used to treat a cardiovascular disorder?
What disorder + how?
α-methyl DOPA
- results in production of α-methyl noradrenaline which has about 1/10 the vasopressor effect normal NA, so is an effective treatment for hypertension
Describe the uptake of catecholamines into synaptic vesicles.
2 important components
It is a secondary active transport, driven by an H+-ATPase pump.
The pump creates a proton gradient which drives VMTA2, a non-specific transporter of biogenic amines.
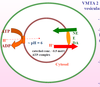
What is the transporter responsible for neurotransmitter uptake into vesicles?
What is its substrate specificity?
VMTA2 - Vesicular Membrane Transporter 2
- 12 TM domain transporter with broad substrate specificity for biogenic amines
What drug disrupts the uptake of catecholamines into synaptic vesicles?
How?
What can it treat?
Reserpine
- irreversibly inhibits uptake via inhibition of VMTA2 + thus depletion of synaptic vesicles (b/c constant leakage is not balanced by uptake)
- can be used to treat hypertension
What psychiatric disorder is associated with disrupted VMTA2 activity?
How is it treated?
Bipolar Disorder
- treated with lithium
(check notes for the mechanism)
What two enzymes are involved in metabolism of noradrenaline?
- Monoamine Oxidase
- COMT - Catechol O-methyl Transferase












