Vascular surgery Flashcards
- Varicose veins are
- A
- RF
- CF
- A saphena varix is a dilatation of the saphenous vein at the saphenofemoral junction in the groin. As it displays a ?, it is commonly mistaken for a femoral hernia; suspicion should be raised in any suspected femoral hernia if the patient has concurrent varicosities present in the rest of the limb. These can be best identified via duplex ultrasound and management is via high saphenous ligation.
- Classification
- Ix
- Rx
- Complications

- tortuous dilated vein
valvular incompetence
deep to the superficial venous system -> venous HTN and dilatation
- Primary idiopathic 98%
Secondary causes: DVT, pelvic masses (e.g. pregnancy, uterine fibroids, and ovarian masses), or arteriovenous malformations (such as Klippel-Trenaunay Syndrome)
- Prolonged standing, Obesity, Pregnancy, FHx
- pain, aching, swelling, itching
skin changes: ulceration, thrombophlebitis or bleeding
o/e: varicosities will be present in the course of the great and / or short saphenous veins
venous insufficiency: oedema, varicose eczema or thrombophlebitis, ulcers (medial malleolus), haemosiderin skin staining, lipodermatosclerosis (tapering of legs above ankles, an “inverted champagne bottle” appearance), or atrophie blanche
- cough impulse
- CEAP Classification
- duplex ultrasound
- Non-Invasive
- Education: avoid prolonged standing, wt loss, increase exercise
- compression stockings if interventional treatment is not appropriate
- four-layer bandaging for venous ulceration
Surgical Treatment
In the UK, patients with varicose veins should be referred to a vascular service if they meet the following NICE criteria:
- Symptomatic primary or recurrent varicose veins
- Lower‑limb skin changes e.g. pigmentation or eczema, thought to be caused by chronic venous insufficiency
- Superficial vein thrombosis (characterised by the appearance of hard, painful veins) with suspected venous incompetence
- A venous leg ulcer (a break in the skin below the knee that has not healed within 2 wks)
The treatment options that are available include:
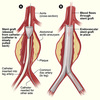
- Vein ligation, stripping, and avulsion – making an incision in the groin (or popliteal fossa) and identifying the responsible, refluxing vein, before tying it off and stripping it away. The surgeon must be aware of surrounding arterial and nervous structures, such as the saphenous and sural nerves.
- Foam sclerotherapy – injecting a sclerosing (irritating) agent directly into the varicosed veins, causing an inflammatory response that closes off the vein (Fig. 3). This is done under ultrasound guidance to ensure the foam does not enter the deep venous system, however this method only requires a local anaesthetic.
- Thermal ablation – which involves heating the vein from inside (via radiofrequency or laser catheters), causing irreversible damage to the vein which closes it off. This is done under ultrasound guidance and also may be performed under local (or general) anaesthetic.
- Typical post-op complications: haemorrhage, thrombophlebitis (important for foam or ablation treatments), DVT (important for any endovenous treatments), disease recurrence, and nerve damage (specifically saphenous or sural nerves).

Deep Venous Insufficiency
- A
- RF
- CF
- DD
- Ix
- Rx
- deep vein thrombosis (DVT) or valvular insufficiency
Causes can be divided into:
- Primary, underlying defect to the vein wall or valvular component: congenital defects and connective tissue disorders
- Secondary, whereby defects occur secondary to damage: post-thrombotic disease, post-phlebitic disease, venous outflow obstruction, and trauma
- increasing age, W>M , pregnancy,
3.
- venous claudication, characterised by a bursting pain and tightness on walking which resolves on leg elevation
- varicose eczema (dry and scaly skin), thrombophlebitis, haemosiderin skin staining, lipodermatosclerosis*, oratrophie blanche**
- renal, hepatic, or cardiac disease
5.
- Doppler ultrasound scan: venous reflux
- bloods LFTs (liver path) U+E (renal) FBC (infection)
- ECHO if any cardiac disease is suspected
- documentation of foot pulses, ABPI
- compression stockings and suitable analgesic control

Thoracic outlet syndrome (TOS)
- What is it?
- How is it divided?
- Brachial plexus and subclavian artery pass through the ?, the subclavian vein anterior to scalenus anterior. The brachial plexus can be compressed between the anterior and middle scalene muscles, or against the 1st rib or a cervical rib, typically with the lower cord being irritated (thus causing symptoms in the ulnar distribution).
Repetitive stress injuries and hyperextension injuries of the neck can cause acute spasm of the scalene muscles, haemorrhage, or swelling of the scalene muscles, which in turn narrows the thoracic outlet. Likewise hypertrophy of these muscles can cause compression in bodybuilders.
Cervical ribs can predispose patients, especially after hyperextension-flexion (whiplash) injury*. Moreover, the presence of a costoclavicular ligament can reduce the costoclavicular space, leading to vTOS, due to positional venous obstruction.
Following trauma, healing processes from clavicular fractures can also cause extra bone formation that compresses the neurovascular bundles.
*The absence of a rib anomaly makes the diagnosis of arterial thoracic outlet syndrome less likely, however the 1st rib can have prominent muscular attachments that compress the artery.
- RF
- CF
- special tests
- Ix
- compression of the neurovascular bundle
hyperextension injuries, repetitive stress injuries (e.g. work-related, particularly when working over the head), or external compressing factors (e.g. poor posture), but can also be secondary to anatomical abnormalities, including that of the 1st rib, an anomalous cervical rib, or bands within the thoracic outlet.
- neurological* (nTOS), venous(vTOS), and arterial (aTOS)
- scalene triangle
- Recent trauma, repetitive motion occupations, athletes*, or anatomical variations are potential risk factors for TOS
*Athletes who compete in swimming, rowing, weightlifting, or any sport that involves the muscles around the neck and shoulder are also at risk, particularly for venous TOS
- worsen with certain movements
paraesthesia and/or motor weakness
deep vein thrombosis and extremity swelling (termed Paget-Schrötter syndrome)
claudicationsymptoms or acute limb ischaemia
- image
7.
- bloods
- CXR: *Over 90 percent of aTOS patients will have a bony abnormality, therefore the absence of rib abnormalities make the diagnosis of aTOS much less likely
- venous and arterial duplex US

Subclavian steal syndrome
- what is it?
- A
- Coronary-Subclavian Steal Syndrome occurs in patients who have undergone an?
- Risk Factors
- Clinical Features
- Investigations:
7.
a. Conservative Management
b. Surgical Management

- syncope or neurological deficits when the blood supply to the affected arm is increased through exercise
2xmales
~left
- atherosclerosis, less common causes: vasculitis, thoracic outlet syndrome (e.g. cervical rib), or complications following aortic coarctation repair
In order to compensate for the increased oxygen demand in the arm, blood is drawn from the collateral circulation, which results in reversed blood flow in the ipsilateral vertebral artery (or less commonly the internal thoracic artery).
- Internal Mammary Artery (IMA) Graft. An increase in oxygen demand in the left arm then steals blood from the IMA leading to cardiac ischaemia.
- Due to the offending lesion tending to be an atherosclerosis, the main risk factors for the condition are increasing age, hyperlipidaemia, hypertension, smoking, and diabetes mellitus.
- arm claudication; pain or paraesthesia, made worse with arm movement.
vertebral artery - wide range of cerebral symptoms: vertigo, diplopia, dysphagia, dysarthria, visual loss, or syncope.
6.
- duplex US scan: retrograde flow in the affected vertebral artery during exercise.
- Routine chest radiograph can aid in assessing for any external compression on the subclavian artery causing the syndrome.
- Definitive CT angiography or MR angiography, which will identify the anatomy of the occlusive lesion in the arm and can also help to assess the cerebral vasculature*
*80% of patients with subclavian steal syndrome will also have additional vascular disease in the rest of the cerebral circulation
7
a. anti-platelet
statin
smoking cessation, weight loss, and optimising diabetic control
b. Occlusions may be treated either through endovascular or bypass techniques. In cases with neurological symptoms, cerebral flow re-establishment must be prioritised over direct treatment of the subclavian stenosis.
Use of percutaneous angioplasty ± stenting (Fig. 3) has reported success rates upwards of 90%, albeit with higher rate of restenosis with worsening disease severity.
Use of bypass should be considered for longer or distal occlusions. Options include carotid-subclavian bypass (5 year patency rates reported at 80%) or axillo-axillary bypass.
- advanced: permanent retrograde flow
Intermittent alternating flow: antegrade flow in the diastolic phase and retrograde flow in the systolic phase

- Hyperhidrosis is defined as sweating in excess of that required for regulation of body temperature.
Sweating is controlled by the autonomic nervous system. Increased sympathetic stimulation from thoracolumbar autonomic fibres stimulate the eccrine (water) sweat glands (rather than the oily apocrine glands) to increase sweat production.
Hyperhidrosis can be divided into:
- Causes:
- CF
- RX
- Primary – idiopathic and usually localised to specific areas e.g. hands, armpits, scalp, or feet, ~ symmetrical distribution. Most cases start in the teenage years yet will improve as the patient gets older; a third of patients have a positive family history
- Secondary – underlying condition/ medication and can present with generalised sweating or focal to specific areas
2.
- Pregnancy or menopause
- Anxiety
- Infections: tuberculosis, HIV, or malaria
- Malignancy, especially lymphoma
- Endocrine disorders: hyperthyroidism, phaeochromocytoma, or carcinoid syndrome
- Medication
- Including anticholinesterases, antidepressants, or propranolol
- Primary:
focal sweating, ~ bilateral and symmetrical, >1/wk, ~onsets before 25yrs, present for >6months to diagnose
Secondary: generalised sweating and in many cases predominantly at night time*features of underlying secondary causes: pyrexia, palpitations, or unexplained weight loss.
*Interestingly, many cases of primary hyperhidrosis will stop during sleep
- lifestyle advice: reducing stress or anxiety, avoiding spicy food, and using absorbant underlayers or armpit pads. Loose fitting clothes of natural fibre and leather shoes can also help.
Anti-perspirants, aluminium chloride (applied at night only) can also be trialled (although this can often cause painful and erythematous skin in the areas applied)
Propantheline is the only anticholinergic agent licenced for use in hyperhidosis. Glycopyrrolate and oxybutynin (also anticholinergic agents) can reduce sweating but not readily available and are often off-license.
- Iontophoresis involves use of a weak electrical current through the area through water soaked sponges
Only a short-term solution, likely works by a combination of blocking sweat glands, disrupting nerves, and making sweat more acidic
- Botulinum toxin (block the nerve supply to the sweat glands)
lasts around 2-6 months and can be repeated, only licensed for underarm but not for hands or feet as this can result in weakness.
- Endoscopic thoracic sympathectomy (ETS) involves causing damage to the thoracic sympathetic ganglion supplying the affected region (most useful for palm and face involvement)
This is a major operation with the risk of damaging other nerves or the lung parenchyama, hence should only be done as a last resort
*Many patients who receive treatment often develop to compensatory sweating in another locations, hence is a side effect that patients must be informed of
Acute limb ischaemia: 6hrs
- A
- CF
- How can we categorise Acute limb ischaemia
- Ix
- Rx
- Long Term Management
- Complications
1.
- Thrombosis in situ (60%)
- Embolisation (30%) (chronic limb ischaemia, AF, recent MI (resulting in a mural thrombus), or a symptomatic AAA (ask about back/abdominal pain) and peripheral aneurysms)
- Trauma (10%), including compartment syndrome
2.
- Pain
- Pallor
- Pulselessness
- Paresthesia
- Perishingly cold
- Paralysis
- when the venous doppler becomes inaudible
4.
- Routine bloods,
- serum lactate (to assess the level of ischaemia),
- thrombophilia screen (if <50yrs without known RF)
- group and save ?AAA
- ECG ?AF
- Bedside Doppler ultrasound scan (both limbs)
- CT angiography
If the limb is considered to be salvageable, a CT arteriogram can provide more information regarding the anatomical location of the occlusion and can help decide the operative approach (such as femoral vs. popliteal incision).
- Initial Management
- high-flow oxygen
- adequate IV access
- therapeutic dose heparin or preferably a bolus dose then heparin infusion should be initiated as soon as is practical.
Conservative Management (Rutherford 1 and 2a SENSORY)
- prolonged course of heparin
- regular assessment to determine its effectiveness -> APPT and clinical review
Surgical Intervention (Rutherford 2b)
Embolic:
- Embolectomy via a Fogarty catheter
- Local intra-arterial thrombolysis*
- Bypass surgery (if there is insufficient flow back)
thrombotic disease:
- Local intra-arterial thrombolysis
- Angioplasty (Fig. 3)
- Bypass surgery
Irreversible limb ischaemia (mottled non-blanching appearance with hard woody muscles) requires urgent amputation or taking a palliative approach.
- regular exercise, smoking cessation, and weight loss as necessary
anti-platelet agent, such as low-dose aspirin or clopidogrel, or even anticoagulation with warfarin or a DOAC.
amputation: OT, PT
7. mortality rate of around 20%
30-day mortality rate following the surgical treatment at 15%
Reperfusion injury; sudden increase in capillary permeability can result in:
- Compartment syndrome
Release of substances from the damaged muscle cells:
- K+ ions causing hyperkalaemia - arrhythmia
- H+ ions causing acidosis
- Myoglobin, resulting in significant AKI
- It is imperative that patients at risk of compartment syndrome are closely monitored and rapidly treated. Electrolyte imbalance due to reperfusion injury requires close monitoring and potentially haemofiltration.

Chronic limb ischaemia
- RF
- CF
- Test
- Leriche syndrome
- Critical limb ischaemia is the advanced form of chronic limb ischaemia.
It can be clinically defined in three ways:
- DD
- state the severities of ankle brachial bp
- Rx

- Smoking
- Diabetes mellitus
- Hypertension
- Hyperlipidaemia
- Increasing age
- Family history
- Obesity and physical inactivity
- intermittent claudication, a cramping-type pain in the calf, thigh, or buttock after walking a fixed distance (the ‘claudication distance’), relieved by rest within minutes.
- Buerger’s test
supine and raising their legs until they go pale and then lowering them until the colour returns (or even becoming hyperaemic). The angle at which limb goes pale is termed Buerger’s angle; <20 degrees indicates severe ischaemia.
- PAD affecting the aortic birfurcation -> buttuck pain , thigh pain and ED
5.
- Ischaemic rest pain for >2wks duration, requiring opiate analgesia
- Presence of ischaemic lesions or gangrene objectively attributable to the arterial occlusive disease
- ABPI <0.5
o/e: the limbs may be pale and cold, with weak or absent pulses.
Other signs: limb hair loss, skin changes (atrophic skin, ulceration, or gangrene), and thickened nails.
6.
- Spinal stenosis (‘neurogenic claudication’)
Typically have pain from the back radiating down the lateral aspect of the leg (tensor fascia lata), often have symptoms on initial movement or symptoms that are relieved by sitting rather than standing
- Acute limb ischaemia
Clinical features that are less than 14 days duration, often presenting within hours.
*Acute-on-chronic ischaemia is a more complex condition whereby there is an acute often embolic event in a patient with previous peripheral arterial disease. These patients are sub-classified as they typically have a longer duration in which the limb is salvageable.
7.
- Normal >0.9
- Mild 0.8-0.9
- Moderate 0.5-0.8
- Severe <0.5
ABPI value >1.2 ?calcification and hardening of the arteries may cause a falsely high ABPI.
8.
- Lifestyle advice (smoking cessation, regular exercise, weight reduction)
- Statin therapy (ideally atorvastatin 80mg OD)
- Anti-platelet therapy (ideally clopidogrel 75mg OD)
- Optimise diabetes control
surgery
- Angioplasty with or without stenting (Fig. 3)
- Bypass grafting, typically used for diffuse disease or in younger patients
- A combination such as surgery to clean a specific lesion allowing access for angioplasty to another region

- The common causes of acute mesenteric ischaemia can be classified into:
- CF
- DD
- Ix
- Rx
- Comp of MI
1.
- Thrombus-in-situ (Acute Mesenteric Arterial Thrombosis, AMAT) 25% Atherosclerosis
- Embolism (Acute Mesenteric Arterial Embolism, AMAE) 50% Cardiac causes* or abdominal / thoracic aneurysm
- Non-occlusive cause (Non-Occlusive Mesenteric Ischemia, NOMI) 20% Hypovolemic Shock, Cardiogenic Shock
- Venous occlusion and congestion (Mesenteric Venous Thrombosis, MVT) <10% Coagulopathy, Malignancy, or Inflammatory Disorders
*Cardiac causes include arrhythmias (e.g. AF), post-MI mural thrombus, or prosthetic heart valve
- generalised abdominal pain, out of proportion
nausea and vomiting 75%
abdomen is often unremarkable*
embolic sources, such as AF, heart murmurs, or signs of previous valvular replacement surgery.
- peptic ulcer disease, bowel obstruction, and symptomatic AAA
- ABG: degree of acidosis and serum lactate, secondary to the severity of bowel infarction.
FBC,
U&Es,
clotting (especially if patient anti-coagulated),
amylase*
LFTs (if the coeliac trunk is affected, ischemia of the liver may cause derangement)
group and save
*Whilst an amylase is commonly measured to exclude pancreatitis as a cause of the abdominal pain, counter-intuitively amylase also rises in mesenteric ischaemia, as well as ectopic pregnancy, bowel perforation, and DKA
Imaging
- CT scan with IV contrast (triple phase scan, with thin slices taken in the arterial phase)
- Arterial bowel ischaemia will initially as oedematous bowel and vasodilatation, before progressing to a loss of bowel wall enhancement* and then to pneumatosis.
*Oral contrast should be avoided in cases of mesenteric ischaemia due to difficulty in assessing for bowel wall enhancement
- perforation warrants an initial AXR and erect CXR; if there is significant suspicion, then a CT abdomen with contrast is indicated.
5.
Initial:
- IV fluids, a catheter inserted, and a fluid balance chart
- confirmed: broad-spectrum antibiotics
- The patient will have a significant acidosis and is at a high risk of developing multiorgan failure, therefore early ITU input to optimise the patient is necessary
Definitive: (location, timing, and severity)
- Excision of necrotic or non-viable bowel, if not suitable for (or able to access) revascularisation
Post-op ICU under sedation, planned for potential relook laparotomy in 24-48 hours; the majority of patients will end up with a (either covering loop or end stoma) and there is a high chance of short gut syndrome.
- Revascularisation of the bowel - removal of thrombus or embolism via radiological intervention; the decision depends on state of the patient, the bowel, and the angiographic appearance of the mesenteric vessels
This is preferably done through angioplasty due to the risk of aortic contamination in open surgery, however open embolectomy is possible either through the CT, SMA, IMA, or the aorta
- bowel necrosis and perforation
Mortality is around 50-80%
short gut syndrome: diarrhea, which can result in dehydration, malnutrition, and wt loss
Peripheral artery aneurysms and visceral artery aneurysms
- aneurysm is defined as
- A, RF, CF
- Ix & Rx
- The most common peripheral artery aneurysms occur in the?
- persistent, abnormal dilatation of an artery above 1.5x its normal diameter.
2.
- largely unknown
- Possible causes: trauma, infection, CT disease (e.g. Marfan’s disease), or inflammatory disease (e.g. Takayasu’s aortitis).
RF: smoking, HTN, hyperlipidaemia, and FHx
CF:
- Asymptomatic (found incidentally)
- Symptomatic, but not ruptured
- Symptomatic secondary to a rupture (stable or unstable)
3.
- CT angiography (gold standard)
- MR angiography (reduce risk of kidney damage)
- US duplex scans can be useful for detection and follow up, but will not help with planning of treatment.
watchful waiting or surgical intervention (endovascular or open)
- popliteal artery and femoral artery
Popliteal Artery (70-80%)
high risk of embolisation, so active management is advised
- Presentation
- o/e
- Ix
- Management
1.
- ~ symptomatically as either acute limb ischaemia (from aneurysm thrombosis or distal emboli) or less commonly with intermittent claudication
- incidentally, e.g. in patients being worked up for AAA repair or awaiting knee replacement. The rupture of popliteal artery aneurysms is rare.
- pulsatile mass will be felt in the popliteal fossa
~ bilaterally
are associated with AAA (20%)
3.
- US duplex scan (rule out Bakers cyst or lymphadenopathy, whilst also looking for signs of aneurysm thrombosis)
- Further imaging will often be via CT Angiogram or MR Angiogram. These imaging modalities allow good anatomical assessment of the aneurysm (useful for operative planning) and in the assessment of distal arteries to assess their patency.
4.
Treat if:
symptomatic popliteal aneurysms, due to risk of embolic events, regardless of their size; if a thrombus is seen on imaging, this should warrant treatment at an early stage.
asymptomatic, but >2cm, popliteal aneurysms should be treated. Otherwise they should be routinely monitored with US scans.
Surgical Intervention (open and endovascular)
- Endovascular repair: stent insertion across the aneurysm and requires a normal calibre artery above and below the aneurysm for the stent to seal in.
Risks: continued aneurysm sac filling through collateral vessels, in-stent thrombosis can occur.
It is done under local anaesthetic and is often the preferred choice in unfit patients
- Surgical repair: ligation or resection of the aneurysm with abypass graft (either a vein from the patient (preferred) or a graft). This can be in the form of an above to below knee popliteal bypass, or through a posterior approach to the knee, opening the aneurysm sac then inserting a tube graft from top to bottom.

types of angiography

Femoral Artery
- There are two major causes for the development of a femoral artery aneurysm:
- Presentation
- Ix and Rx
Visceral Artery Aneurysms
- The visceral arteries most commonly affected by aneurysm formation are the
- Investigation and Management
Hepatic artery aneurysms are the second most common of the visceral aneurysms, accounting for around 20% of cases.
Percutaneous instrumentation is associated in 50% of cases, yet the remainder of cases may be from trauma, degenerative disease, or post-liver transplant (false aneurysms forming around vessel anastomoses).
Presentation
Most cases are usually asymptomatic, yet stable symptomatic cases can often present with vague RUQ or epigastric pain; jaundice can occur if there is any biliary obstruction.
Investigation and Management
Much like splenic aneurysms, the mainstay of investigation for hepatic artery aneurysms is byCT Angiography or MR Angiography.
First line management is endovascular repair; this is best done with embolization or stent grafts, once the patient is haemodynamically stable, in those with suitable anatomy (an open repair may be advised in the unstable patient or with unsuitable anatomy).
Renal Artery
A renal artery aneurysm is often found incidentally and is asymptomatic. However, in symptomatic cases, patients may present with haematuria, resistant hypertension, or loin pain (including those with renal infarction).
Investigation and Management
Renal artery aneurysms should also be investigated by CT Angiography or MR Angiography.
Mainstay of treatment is via endovascular repair. A stent can be inserted easily if the aneurysm is affecting the main renal artery, otherwise if it is affecting the hilum, endovascular repair with coils and self-expanding stents may be used.
1.
- Percutaneous vascular interventions
- IVDU
2.
either thrombosis, rupture, or embolisation of the aneurysm. In IVDU cases, concurrent infection may also be present.
As a consequence, patients will present with varying degrees of claudication or acute limb ischaemia, but often with no symptoms other than a swelling in the groin.
3.
- US duplex scan
- CT Angiography or MR Angiography
Open surgical repair (endovascular repair is rarely performed)
4. splenic artery (60%), hepatic artery, and renal artery.
RF: F, multiple pregnancies, portal HTN, and pancreatitis or pancreatic pseudocyst formation
Those that are symptomatic will present with a vague epigastric or LUQ pain. Those that rupture will present with severe abdominal pain and haemodynamic compromise.
- splenic artery aneurysms is by CT Angiography or MR Angiography.
Rx endovascular repair (1st); embolisation or stent grafts, once the patient is haemodynamically stable (an open repair may be advised in the unstable patient)
- abdominal aortic aneurysm (AAA) is defined as a
- A & E
- CF
- Screening
- Ix

- dilatation of the abdominal aorta greater than 3cm
- unknown. Possible causes include atherosclerosis, trauma, infection, connective tissue disease (e.g. Marfan’s disease, Ehler’s Danlos, Loey Dietz), or inflammatory disease (e.g. Takayasu’s aortitis).
Risk factors for AAA include smoking, hypertension, hyperlipidaemia, family history, male gender, and increasing age. Diabetes mellitus is a negative risk factor for AAA (the mechanism for this is still poorly understood).
3.
- Abdominal pain
- Back or loin pain
- Distal embolisation producing limb ischaemia
- Aortoenteric fistula
O/e: pulsatile mass can be felt in the abdomen (above the umbilical level), and rarely, signs of retroperitoneal haemorrhage may be evident.
- national abdominal aortic aneurysm screening programme (NAAASP) offer an abdominal US scan for all men in their 65th year
5.
USS
follow up CT scan with contrast when at threshold diameter of 5.5cm. This provides more anatomical details in order to determine suitability for endovascular procedures.
*An AXR is not indicated as it will only rarely show an AAA if there is significant calcification of the arterial wall.
A patient with a ruptured AAA may present with pain (abdominal, back, or loin) and a degree of shock or syncope, as discussed below.
4.
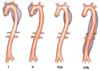
- Draw images for the endovascular leaks in AAA
- The main complication of AAA is rupture, as discussed below. Other less common complications include:
- Management
Any suspected AAA rupture warrants immediate
- image
- Retroperitoneal leak
Embolisation
Aortoduodenal fistula
The risk of AAA rupture increases exponentially with the diameter of the aneurysm*, but the risk is also increased by smoking, HTN, and female gender.
An AAA rupture can present with abdominal pain, back pain, syncope, or vomiting*. On examination they will typically be haemodynamically compromised, with a pulsatile abdominal mass and tenderness.
Around 50% patients present with the ‘classic triad’ of ruptured AAA (flank or back pain, hypotension, and a pulsatile abdominal mass).
*20% of AAA ruptures will rupture anteriorly into the peritoneal cavity (which are associated with a very poor prognosis), whilst 80% rupture posteriorly into the retroperitoneal space
3.
- high flow O2
- IV access (2x large bore cannulae)
- urgent bloods taken (FBC, U&Es, clotting)
- crossmatch for minimum 6U units
- Aim to keep the BP ≤100mmHg (termed ‘permissive hypotension’, preventing excessive blood loss). As long as the patient is cerebrating, the BP is generally adequate.
- transferred to the local vascular unit, with the vascular registrar, consultant, anaesthetist, theatre, and blood transfusion lab informed.
If the patient is unstable, they will require immediate transfer to theatre for open surgical repair
If the patient is stable, they will require a CT angiogram to determine whether the aneurysm is suitable for endovascular repair*
*The IMPROVE trial randomised patients suitable for EVAR or open AAA repair, showing no 30 day or 1 year difference in survival, but an increase in patients returning to their own homes, short hospital stay, and lower cost in the EVAR cohort. The 3 year results show a significant survival benefit for EVAR, particularly when performed under local anaesthesia

AORTIC DISSECTION (tear in the intimal layer)
- Acute vs chronic
- E
- Aortic dissections are classified anatomically by two systems, DeBakey and Stanford:
- RF
- CF
- DD
- complications
- Investigations
Imaging
- Rx
- acute (when diagnosed ≤14 days)
chronic (when diagnosed >14 days)
- M>F
CT disorders, and have a peak onset between 50-70yrs
Anterograde dissections propagate towards the iliac arteries
Retrograde dissections propagate towards the aortic valve
*Retrograde dissections can result in prolapse of the aortic valve, bleeding into the pericardium, and cardiac tamponade
- DeBakey Classification:
Type I – originates in the ascending aorta and propagates at least to the aortic arch
They are typically seen in patients under 65yrs and carry the highest mortality, quoted at 1% per hour in the acute setting
Type II – confined to the ascending aorta
~ elderly patients with atherosclerotic disease and hypertension
Type III – originates distal to the subclavian artery in the descending aorta
Further subdivided into IIIa which extends distally to the diaphragm and IIIb which extends beyond the diaphragm into the abdominal aorta
Stanford Classification
Divides aortic dissection into two groups, A and B:
Group A – includes DeBakey Types I and II and involves the ascending aorta and can propagate to the aortic arch and descending aorta; the tear can originate anywhere along this path
Group B – dissections do not involve the ascending aorta and include DeBakey Type III
4.
- Hypertension
- Atherosclerotic disease
- Male gender
- Connective tissue disorders* (typically Marfan’s syndrome or Ehler’s-Danlos syndrome)
- Biscuspid aortic valve
- tearing chest pain,
radiating through to the back
tachycardia, hypotension*, new aortic regurgitation murmur, or signs of end-organ hypoperfusion (such as reduced urine output, paraplegia, lower limb ischaemia, abdominal pain secondary to ischaemia, or deteriorating conscious level).
6.
- Myocardial infarction – classically crushing and central chest pain, with signs of cardiac ischaemia on ECG and / or raised serum troponin levels
- Pulmonary embolism – dyspnoea will be a prominent feature and an ABG will demonstrate hypoxia, confirm with CTPA or V/Q scan
- Pericarditis – classically pleuritic chest pain, with the ECG showing diffuse ST elevation, as well as potential pericardial rub on auscultation
- Musculoskeletal back pain – the patient will not present with systemic signs of shock and will be tender to palpation of the chest wall or paraspinal muscles
- Baseline blood tests (FBC, U&Es, LFTs, troponin, coagulation)
crossmatch of at least 4 units
arterial blood gas
ECG should also be performed to exclude any cardiac pathology.
CT angiogram to diagnose aortic dissection as (first line) This will also allow classification, establish the anatomy of the dissection, and assist surgical planning.
A transoesophogeal ECHO can also provide useful information but is operator dependent.
8.
high flow oxygen
IV access (x2 large bore cannulas); fluid resuscitation should be done cautiously*.
*In the setting of a rupture, then the target pressure should be sufficient for cerebral perfusion only. In the setting of an uncomplicated dissection then the target systolic pressure should be kept below 110mmHg systolic.
Stanford Type A dissections should be managed surgically in the first instance and carry a worse prognosis than Type B dissections. Any uncomplicated Type B dissections can usually be managed medically.
Following initial management, all patients need lifelong antihypertensive therapy and surveillance imaging*, due to the high risk of developing further dissection or other complications.
*Imaging would usually be at 1, 3, and 12 months post-discharge, with further scans at 6-12 month intervals thereafter depending on the size of the aorta.
Type A Dissections surgically
transfer to a cardiothoracic centre
removal of the ascending aorta (with or without the arch) and replacement with synthetic graft. If the dissection has damaged the suspensory apparatus of the aortic valve, this will also require repair.
Any additional branches of the aortic arch that are involved will require reimplanation into the graft (i.e. brachiocephalic artery, left common carotid artery, left subclavian artery), with long Type A dissections involving the descending and possibly abdominal aorta may require staged procedures.
Type B Dissections medically
- control hypertension with intravenous beta blockers (labetalol) (or calcium channel blockers as second line therapy). The aim of this therapy is to rapidly lower the systolic pressure, pulse pressure, and pulse rate to minimise stress of the dissection and limited further propagation.
- endovascular repair is not recommended due to the risk of retrograde dissection
- Surgical intervention in Type B dissections is only warranted in the presence of certain complications: rupture, renal, visceral or limb ischaemia, refectory pain, or uncontrollable hypertension.
Type B dissections can go on to be chronic, with continued leakage into the dissection, even if a stent has been placed. The most common complication of chronic disease is the formation of an aneurysm. These present further surgical problems, with endovascular repair offering a better survival chance.
- Aortic rupture
Aortic regurgitation
Myocardial ischaemia
Secondary to coronary artery dissection
Cardiac tamponade
Stroke or paraplegia
Secondary to cerebral artery or spinal artery involvement
THORACIC AORTIC ANEURYSM
- Can involve
- E
- A
- RF
- cf:
- pre-op testing
- Rx
- What is the surgical intervention criteria
- ascending aorta or aortic root (60%), aortic arch (10%), descending aorta (40%), or thoracoabdominal aorta (10%) segments*
- less common than abdominal aortic aneurysms, they are associated with high mortality.
- degradation of the tunica media
The main causes of thoracic aneurysm are:
- Connective tissue diseases e.g. Marfan’s syndrome or Ehlers-Danlos syndrome.
- Bicuspid aortic valve, inc risk of thoracic aortic aneurysms is seen in patients with Turners syndrome (from increased risk of bicuspid aortic valve)
- Trauma
- Aortic dissection
- Aortic arteritis, e.g. Takayasu Arteritis
- Tertiary syphilis
- family history*, hypertension, atherosclerosis (specifically descending aneurysms), smoking, high BMI, male gender, and advancing age.
- asymptomatic and are found incidentally
if symptomatic, the most common presenting complaint is pain
Ascending aorta - Anterior chest
Aortic arch - Neck
Descending aorta - Between the scapulae
Other symptoms of thoracic aneurysms include:
Back pain – secondary to spinal compression by descending or thoracoabdominal aneurysm
Hoarse voice – from damage to the left recurrent laryngeal nerve in arch aneurysms
Distended neck veins – from SVC compression
Symptoms of HF – from involvement of the aortic valve
Dyspnoea or cough – secondary to tracheal or bronchial compression
- routine bloods (FBC, U&Es, clotting) with a group and save, ECG, and CXR performed
CXR demonstrating a widened mediastinal silhouette, an enlarged aortic knob, and possible tracheal deviation. However, a radiograph is not sensitive enough to make the definitive diagnosis and further imaging is required
CT chest scan with contrast is the preferred imaging modality for thoracic aneurysms, providing sufficient detail to ascertain the level and the size of the aneurysm*.
TOE can be used to good effect to further detect any concurrent aortic insufficiency or dissection; TOE should form part of the routine assessment of patients with Marfan’s disease and suspected thoracic aortic disease.
CT angiography or MR angiography can overcome this issue
7.
Medical management: statin and antiplatelet therapy
Blood pressure should be controlled and smoking cessation is imperative.
*There is some evidence that suggests beta-blocker therapy in patients with a Marfan’s related thoracic aneurysm can slow the rate of growth, but this has not yet been extrapolated into otherwise healthy patients.

Describe some NOACs



