Urea Cycle Flashcards
(23 cards)
What is the fate of the carbon skeleton of amino acids?
What is the fate of the NH4+ of amino acids?
How are these two processes connected?
- Carbon skeleton
- citric acid cycle
- NH4+
- Urea cycle
- Interaction
- Aspartate-arginino-succinate shunt of citric acid cycle
Where in the body are most amino acids metabolized?
liver
What is the human excretory form of nitrogen?
urea

What four amino acids play a major role in connecting the urea cycle to the citric acid cycle?
What is an important characterisitc about these animo acids?
glutamate
glutamine
alanine
aspartate
They are all easily converted to citric acid cycle intermediates
The urea cycles uses nitrogen from what two places to produce urea?
First nitrogen can come from any amino acid (NH4+)
Second nitrogen comes from aspartate
What are hte 4 fates of the amino acid nitrogen?
- Transamination reactions
- Removal as ammonia
- Role of Glutamate
- Role of alanine and glutamine
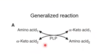
What is the general tranamination reaction?
What amino acid is commonly involved?
What two amino acids do not undergo transamination?
- Amino group from one amino acid is transferrd to another
- converting an alpha-keto acid to an amino acid and an amino acid to a alpha-keto acid
- readily reversible & uses PLP as cofactor
- alpha-ketoglutarate and glutamate are usually one of the pairs
- All amino acids EXCEPT: lysine and threonine
PLP is derived from what vitamin?
vitamin B6
Cells in the body and bacteria in the gut releast nitrogen from amino acids in what form?
Which reactions are reversible & which are irriversible?
What type of reaction occurs with serine & threonine?
- ammonium ion (NH4+)
- source for urea cycle
- brain & muscle: glutamate transfers amino group to oxaloacetate to form aspartate- release fumerate & NH4+
- All of the reactions are irreversible
- EXCEPT glutamate dehydrogenase (GDH)
-
Dehydratase reactions produce NH4+ from serine and threonine
- require PLP

In what form can nitrogen cross cell membranes?
In what form can it no longer freely diffuse?
ammonia (NH3)
once in the interstitial space, it forms NH4+ and can no longer freely diffuse
What is special about asparagine and glutamine?
in addition to the nitrogen availble in their skeleton, they have an additional nitrogen in their side chain
Describe the glutaminase reaction in the kidney
glutaminase acts on glutamine, forming glutamate and NH4+
this forms salts with metabolic acids
What is the difference between deamidation and deamination?
- deamidation
- The conversion of glutamine or asparagine to glutamate or aspartate acid via transamidase or deamidase.
- deamination
- The removal of an amino group from a compound
What are the 3 enzymes that can fix ammonia into an organic molecule?
glutamate dehydrogenase
glutamine synthetase
carbomoyl phosphate synthetase I
What enzymes and cofactors are required to convert glutamate to alpha-ketoglutarate
glutamate dehydrogenase
NAD(P)+
Releases: NAD(P)H and NH4+
readily reversible reaction
What is the important role that glutamate plays in the metabolism of amino acids?
It is involved in both synthesis and degradation of amino acids

Describe the role of glutamate in urea production
- when amino acids are degraded and urea is formed, glutamate collects nitrogens from other amino acids via transamination reactions
- then that nitrogen is released from glutamate via glutamate dehydrogenase and it is able to enter the urea cycle as ammonium
- Glutamate can also undego transamination to produce aspartate that will contribute a nitrogen to the urea cycle
Where are urea cycle enzymes most active?
why is this important to know?
the liver
because there must be a way to transport the nitrogen from amino acid metabolism to the liver
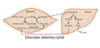
Describe the glucose-alanine cycle
- Alanine is primarily exported by muscles because the muscles are metabolizing glucose through glycolysis & therefore pyruvate is available to be transaminated to alanine
- Alanine serves as a nitrogen carrier, and travels to the liver where it is degraded into carbon (recycled- gluconeogenesis) and nitrogen (enters urea cycle)
- movement of glucose from liver to muscle is called, glucose-alanine cycle
Describe how glutamine can act as a nitrogen carrier
- muscles convert alpha-ketoglutarate to glutamate via glutamate dehydrogenase (NH4+ + NADPH)
- this glutamate is then converted to glutamine via glutamine synthetase (NH4+ + ATP)
-
Glutamine then serves as a nitrogen carrier and traves to the liver where it is degraded to glutamate and NH4+ via glutaminase
- that glutamate is converted to alpha-alphaketoglutarate & NH4+ via glutamate dehydrogenase
- both NH4+ produced enter the urea cycle

What does it mean to be in nitrogen balance?
How is this accomlished?
the amount of nitrogen ingested equals the amount excreted
excreatory product is urea (produced in liver)
Why is it important to excrete nitrogen?
How is it present in the body?
Ammonia is toxic to brain & nervous system
there is a little ammonia NH4+ present in the blood (30-60 micromolar)
It is also present in the blood vi carriers: alanine and glutamine
What are the 5 steps to the urea cycle?
Include location where each step takes place, enzymes, products & if ATP is required for the reaction
- Mitochondria
- (1) Ammonium will react with bicarbonate via enzyme carbamoyl phosphate synthetase I (and use of 2 ATP) to produce Carbamoyl phosphate
- (2) Carbamoyl phosphate is acted on by ornithine transcarbamylase and use an ornithine to produce citruline
- Citruline will leave the mitochondria
- Cytosol
- Citruline will become part of the urea cycle
- (3) Citruline acts with aspartate via arginosuccinate synthetase (use of 1 ATP) to produce arginosuccinate
- (4) Arginosuccinate is acted on by arginosuccinate lyase which will release a molecule of fumerate and produce arginine
- (5) Arginine is acted on by arginase, which release urea (excreted in the urine) and produces ornithine
- ornithine again enters the mitochondria



