Translation Flashcards
(31 cards)
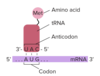
what is a codon
3 consecutive nucleotides in mRNA that codes for one amino acid
what is an anticodon?
3 consecutive nucleotides in tRNA that is complimentary to the nucleotides of a specific codon
what is start codon?
- 3 consecutive nucleotides in mRNA that indicates initiation of translation
-
AUG:
- codes for methionine in eukaryotes
- formylmethionine in bacteria
- the first codon of mRNA translated by ribosome
what is stop codon
- 3 consecutive nucleotides in mRNA that indicates termination of translation
- UAA, UAG or UGA (GUG, UUG are also stop codons in bacteria)
- not translated by ribosome
- binds to release factors instead of tRNA
how do codons code for amino acids?
- codon has complimentary anticodon on tRNA
- codon codes for the amino acid through binding to complimentary anticodon on tRNA
- tRNA act as adaptor between codon and the amino acid it codes for
what is a ribosome
- large ribonucleoprotein that has petidyle transferase activity
- active site composed of RNA => it’s a ribozyme
- translate mRNA transcript into amino acid sequence
the structure of ribosome?
- large subunit (LSU): peptidyl transferase active site
- small subunit (SSU): mRNA binding
- peptidyle transferase active site has three sub sites:
- A (aminoacyl tRNA) site: where tRNA with single amino acid binds
- P (peptidyl tRNA) site: where tRNA with peptide chain attached binds
- E (exit) site: where tRNA leaves after its peptide chain is transferred to the next tRNA

what is tranlation initiated by?
initiation factors (IF)
(eukaryotes) how do initiation factors initate translation?
- eIF = eukaryotic initation factor
- SSU binds to mRNA
- eIF4 untangles mRNA
- eIF3 dissociates LSU from SSU => expose active site
- eIF1 blocks A site => prevents tRNA binding to A site
- eIF2 binds to tRNAimet, guides it to P site, expending one GTP => releases IFs, allow LSU to bind
How does SSU recognise mRNA in eukaryotes?
binds to 5’ cap and finds the first AUG after that
How does SSU recognise mRNA in prokaryotes?
SSU recognise the combination of shine dalgarno sequence (SDS) and AUG
prokaryotic ribosomes have anti-SDS at 3’ end of SSU RNA, binds to SDS
why does bacteria have to couple start codon with SDS (shine dalgarno sequence)
- initiating AUG is distinguished from other AUGs by SDS
- prevent ribosome from mistakening internal methionine codon or out of phase codon as start codon
what is tRNAimet
- initatior tRNA that has amino acid methionine bound to its 3’ end
what is tRNAi
- initiator tRNA
- tRNAi is different to tRNA involved in elongation
what amino acid is eukaryotic and archaic tRNAi charged with
methionine => tRNAimet
what amino acid is bacteiral tRNAi charged with
formylmethionine => tRNAifmet
what happens to the starting methionine after translation
starting methionine is differnt to internal methionine, it is cut off after translation
what enzymes charges tRNA with amino acid
- aaRS = aminoacyl-tRNA synthetase
- catalyses formation of ester bond between 3’ end of tRNA and amino acid
- requires hydrolysis of ATP and produces a high energy bond between the tRNA and amino acid
- energy used in protein synthesis to link amino acid to the growing polypeptide chain

what is meant by the redundancy of the genetic code?
- most amino acids are specified by more than one mRNA codon
- one amino acid can attach to several differnt tRNAs
what factors aids the elongation of translation?
elongation factors (EF)
How does elongation factors aid elongation?
- EF1 binds to tRNAaminoacyl , brings it to A site, expends a GTP then detaches
-
EF2 shunts ribosome along one codon and triggers LSU’s petidyl-transferase activity, expends a GTP:
- peptide bond formation between peptide chain on P site tRNApeptidyl and the amino acid on A site tRNAaminoacyl => one more amino acid is added to the peptide chain
- the tRNA that had peptidyl group bound to it leaves via E site
- the tRNAaminoacyl becomes tRNApeptidyl, is moved into P site
what is the equivalent of EF1 in bacteria?
EF-Tu
what is the equivalent of EF2 in bacteria?
EF-G
How does termination of translation occur?
- stop codon recognised by release factor = tRNApetidyl hydrolase
- hyrolyses petidyl group off tRNA
- similar shape to tRNA => it’s a molecular mimic, allows it to bind to the same active site that tRNA binds to
- peptide then leaves via a channel in LSU


