Topic 6 - Bioenergetics Flashcards
(158 cards)
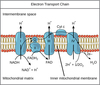
Key roles of mitochondria?
- A central player in bioenergetics
- Produce 90% of cellular energy
- Regulate cellular redox states
- Calcium homeostasis
- Produce reactive oxygen species
- Synthesis and degrade high energy biochemical intermediates
- regulate cell death –> programmed cell death
What are the requirements for the existence of life?
- Information –> Nucleotides/DNA
- Energy –> ATP
Both of these requirements are needed because life is improbable as living systems are highly ordered –> doesn’t follow 2nd law of thermodynamics.
Information specifies what form the order should take and energy drives the reactions and processes for it to occur.
How do we define energy?
In biochemistry –> The ability to cause specific change.
What are the different categories of change?
- Synthetic –> changes in bonds –> i.e. to make macromolecules.
- Mechanical –> muscle contraction/flagellum/ribosome movement
- Concentration –> change in concentration across a membrane.
- Electrical –> movement of ions across membranes (neurons)
- Heat –> heat production –> maintain temperature.
What are the different sources of energy?
- Solar radiation –> plants use to photosynthesize –> produce nutrients –> consumed by humans.
- Electrical discharge
- Chemical energy
What are the two ways chemical energy can be released?
- Fermentation –> glycolysis
- Oxidation –> respiration
Definition of thermodynamics?
The laws governing energy transactions that accompany most processes and reactions.
Definition of bioenergetics?
Applied thermodynamics –> Application of thermodynamic principles to reactions and processes in biological worlds.
Difference between classical and non-classical thermodynamics?
- Classical –> Equilibrium reactions in closed systems.
- Non-classical –> Does not occur at equilibrium, takes into account time and occurs in open systems.
Note –> Living systems do not operate at equilibrium –> hence we use non-classical thermodynamics.
What do living systems do when they consume nutrients? (In terms of enthalpy and entropy)
Living systems consume high enthalpy/low entropy nutrients and convert them to low enthalpy/high entropy products.
- Free energy from this is used to do work and thus allows organisms to create a high degree of organisation.
Why is it important for an organism to stay in a non-equilibrium state?
- In order to perform useful work
- Equilibrium processes cannot be directed –> all regulatory functions require a non-equilibrium state.
- A non-equilibrium state is Inherently unstable –> gives rise to biochemical reactions –> allow us to degrade.
Are regeneration and degradation constantly occurring?
Happens all the time –> requires a constant influx of energy.
What is one thing that always occurs in non-equilibrium reactions?
In non-equilibrium reactions, something must always flow.
Flow –> change in spatial distribution of something (matter/heat/electrical charge/etc.)
Note –> All these flows are conjugate to their thermodynamic force.
Examples
- Flow of matter in diffusion –> driven by conc. gradient
- Transport of heat –> temperature gradient
- Migration of electrical charge –> current –> due to voltage gradient
- Chemical reaction –> difference in chemical potential.
Can a thermodynamic force also promote a non-conjugate flow?
Yes, a thermodynamic force may also promote a non-conjugate flow.
For example, a concentration gradient of matter can give rise to a chemical reaction (or heat or electrical current)
I.e. ATP synthase uses an electrochemical gradient to create chemical bonds.
What is energy transduction?
Energy transduction –> when a thermodynamic force stimulates a non-conjugate flow.
What is metabolism?
Metabolism is the study of energy flow in biological systems –> overall process by which living systems acquire and utilize free energy.
Definition of exergonic reaction?
An exergonic reaction refers to a reaction where energy is released –> ΔG is negative.
Definition of endergonic reaction?
A chemical reaction in which energy is absorbed.
ΔG > 0
What are the names of the different molecules after ATP hydrolysis?
ATP –> ADP –> AMP –> Adenosine.
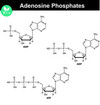
Why does the hydrolysis of ATP release so much energy?
Phosphoanhydride bond is a high energy bond which releases a lot of energy.
What is the ΔG for the hydrolysis of one phosphate and for two phosphates?
H2O + ATP —-> ADP + Pi (ΔG = -30.5 KJ mol-1)
ATP + H2O —-> AMP + PPi (ΔG = -45.6 KJ mol-1)
What factors impact the ΔG of ATP hydrolysis?
Depends on pH, ionic strength and [Mg2+]
Is it possible to have different nucleotide triphosphates?
Yes, some biosynthetic reactions are driven by the hydrolysis of other nucleoside triphosphates –> I.e. GTP.
What are phosphoryl transfer reactions?
Refers to the transfer of a phosphate from one compound to another.
R1-O-PO32- + R2-OH —> R-OH + R2-O-PO32-
A very important reaction for biosynthesis of proteins, nucleic acids and carbohydrates.